- Volume 58 , Number 1
- Page: 50–7
Pathogenesis of route-related variation in t-suppressor response on immunization with mycobacteria
ABSTRACT
The route of immunization was observed to play a significant role in deciding the outcome of immunization with killed mycobacterial vaccines. Earlier we reported that the slow growers were immunogenic by both the intraperitoneal (i.p.) and intradermal (i.d.) routes. In contrast, the rapid growers were immunogenic by the i.d. route only. Both rapid and slow growers generated the classical, antigen-specific Lyt-2 positive, T-cell-mediated suppression after i.p. immunization but not after i.d. immunization. Thus, in the case of the slow growers, T-cellmediated suppression was only a component of the immune response generated after i.p. immunization. In contrast, in the case of Mycobacterium vaccae and the other rapid growers, the T-cell-mediated suppression was the predominant response with i.p. immunization. The T-cell-mediated suppression generated by i.p. immunization exhibited crossreactivity, the spectrum of which was dependent upon the dose of the immunization.
RÉSUMÉ
Il est apparu que la voie d'immunisation joue un rôle important dans le résultat de cette immunisation lorsqu'elle est pratiquée avec des vaccins mycobactériens tués. On a rapporté précédemment que les bacilles à croissance lente étaient immunogènes, tant par voie intrapéritonéale que par voie intradermique. Par contre, les bacilles à croissance rapide ne sont immunogènes que par voie intradermique. Tant les bacilles à croissance rapide que ceux à croissance lente ont entraîné après immunisation intrapéritonéale la suppression bien connue des mécanismes immunologiques sous la dépendance des celules-T positives pour l'antigène spécifique Lyt-2. Cette suppression n'a pas été observée après immunisation par voie intradermique. Dès lors, lorsqu'il, s'agit des bacilles à croissance lente, la suppression impliquant les cellules-T ne constitue qu'un élément de la réponse immune induite par l'immunisation intrapéritéonéale. Par contre, dans le cas de Mycobacterium vaccae, et des autres bactéries à croissance rapide, la suppression des cellules-T constituait Ia réponse principale après immunisation intrapéritéonéale. La suppression induite par l'immunisation intrapéritéonéale produit des phénomènes de réactivité croisée dont le spectre dépend de la dose utilisée pour cette immunisation.
RESUMEN
Se observó que la ruta de inmunización juega un papel importante en el tipo de respuesta mediada por células T generada por administración de vacunas micobacterianas muertas. Se encontró que las micobacterias de lento crecimiento son inmunogénicas cuando se administran tanto por la ruta intraperitoneal (i.p.) como por la intradérmica (i.d.); las micobacterias de crecimiento rápido solo fueron inmunogénicas por la ruta i.d. El estado de falta de respuesta que sigue a la inmunización por vía i.p. con Mycobacterium vaccae pudo corregirse por tratamiento de los ratones con poli I: poli C, o con indometacina, antes de la inmunización. Tanto el poli I: poli C, un inductor de interferon, como la indometacina, un inhibidor de prostaglandinas, se sabe que incrementan la expresión de glicoproteínas del complejo mayor de histcompatibilidad. Puesto que éstas son tan importantes en la preparación del antígeno, se concluyó que la incapacidad de los ratones para responder al M. vaccae por la ruta i.p. se debe, probablemente, a la defectuosa presentación de los antígenos bacterianos por los macrófagos peritoneales. Estos hallazgos son significantes porque se ha reportado que M. leprae es antigenicamente similar a M. vaccae. y la respuesta de los ratones a la inmunización i.p. con estas 2 micobacterias es también muy similar.
Mycobacterial diseases such as leprosy and tuberculosis are characterized by phases of illness associated with an immunological nonresponder state (6, 12). There is also evidence that the nonresponder states are associated with immune suppression, particularly so in lepromatous leprosy (9, 10). However, the pathogenesis of the above phenomenon is still not clear. Some investigators have suggested a genetic defect in antigen presentation linked to major histocompatibility complex (MHC) encoded DR antigens (18, 19). Based on the outcome of immunization of mice with Mycobacterium leprae by different routes, others (13) have suggested that the immune suppression in lepromatous leprosy could be related to the route of infection.
By employing cultivable mycobacteria, we have investigated the role of the route of immunization in immunogenicity. It has been shown (15, 16) that organisms such as M. vaccae are nonimmunogenic by the intraperitoneal (i.p.) route of immunization because the peritoneal cells cannot present their antigens adequately to generate a T-cell response. However, inadequate presentation can only partially explain the pathology of the nonresponder state, because the currently accepted pathology of the nonresponder state also postulates immune suppression (9, 10). Whether such a suppression is also related to the route of infection and, if so, whether the phenomenon is due to differences in antigen-presenting cells involved at different sites is still far from clear.
MATERIALS AND METHODS
Organisms. M. tuberculosis H37Rv (NCTC 7416), M. avium (nonchromogen NCTC 8559) and M. phlei (rapid grower NCTC 8151) were purchased from the National Collection of Type Cultures, London. M. vaccae (rapid grower) was kindly supplied by Dr. J. L. Stanford of Middlesex Hospital, London.
Mice. BALB/c and Swiss white mice 4 to 6 weeks old obtained from the National Institutes of Health, Bethesda, Maryland, U.S.A., and bred at the Cancer Research Institute and Haffkine Biopharmaceutical Corporation Ltd., Bombay, India, were used in the study.
Immunization. Four- to six-week-old cultures of slow-growing mycobacteria and 1-week-old cultures of M. vaccae and M. phlei, on the medium of Doub and Youmans (3), were killed by irradiation .The bacterial cells were then harvested and washed in phosphate buffered saline (PBS), pH 7.2. The washed bacterial cells were pelleted by centrifugation at 2200 × g × 1 hr. The supernatants were discarded, and the washed cell pellets were suspended in PBS to give a 1% (v/v, ≈ 109bacilli/ml) suspension. The required number of bacilli were suspended in 0.5 ml for intraperitoneal (i.p.) immunization and 0.05 ml for intradermal (i.d.) immunization.
Preparation of sonic extract. Four- to sixweek-old cultures of slow-growing mycobacteria and 1-week-old cultures of rapidgrowing M. vaccae, on the medium of Doub and Youmans (3), were killed by 2.4 Mrad gamma radiation in a 60Co source. The killed cells were washed with PBS, disrupted in a sonicator (Branson Sonifier, B-30), and spun at 50,000 × g × 1 hr. The supernatant, i.e., the sonic extract, was employed as the "test antigen." The protein content of the test antigen was estimated by the method of Lowry, et al. (7).
Delayedtype hypersensitivity (DTH) test. The DTH test was done by the method of Gray and Jennings (5). Sonicates containing 50 μg of protein in 0.03 ml volume were injected into the hind foot pad and measurements of footpad thickness were taken before and 24 hr and 48 hr after injection. All ofthe sonicates were also tested in nonimmune mice as controls. The results were expressed as corrected footpad enlargement (CPE), which is the difference between the footpad enlargements caused by a test sonicate in the immunized and control mice, simultaneously tested. The statistical Student's t test was done on CPE values by comparing with zero.
Depletion of T cells, DTH effectors, and T suppressors. The depletion of T cells, DTH effectors and Tsuppressor cells from lymphoid cell suspensions was done by treatment with monoclonal antiThy-1.2, anti-L3T4, and antiLyt-2.2 antibodies and complement as described earlier (4). The monoclonal antibodies were kindly provided by Dr. C. S. Henney of Immunex Corporation, Carson City, Nevada, U.S.A.
Lymphoproliferation test. A lymphoproliferation test (LT) was carried out employing the standard microculture technique (11). Briefly, each well of a microtiter plate (Nunc, Denmark) received 2 × 105cells in 0.2 ml of the culture medium RPMI 1640, containing 2.0 raM glutamine, 10% fetal calf serum (FCS), 1% antibiotic antimycotic (AA; GIBCO, Grand Island, New York, U.S.A.), and 10 μg/ml of sonic extract of the mycobacterial culture under test. The cultures were pulsed 120 hr later with 0.5 μCi of 3H-thymidine (BARC, India, sp. act. 2 Ci/mMol) for a period of 16 hr, harvested, and incorporation ofthe label in the cellular DNA was assayed. All tests were run in quintuplicate.
RESULTS
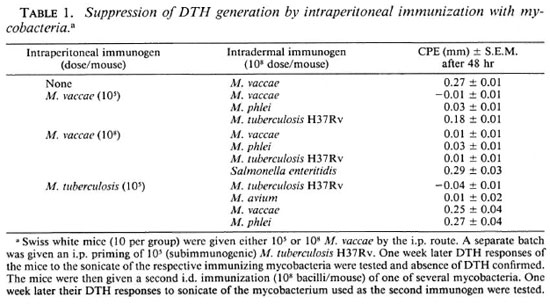
Generation of DTH suppressing T-cell responses after i.p. immunization. The Swiss whitemiceweregivenvaryingdoses(105-109) of M. vaccae bythei.p.route later they were given a second immunization with 108 M. vaccae by the i.d. route, shown earlier to be immunogenic (16), and the DTH to its sonicate was tested. Immunization of the mice by the i.p. route prior to i.d. immunization completely suppressed the generation of a DTH response by the latter. The otherrapid growers tested, such as M. phlei, showed a similar suppressive effect of i.p. immunization on the subsequent i.d. immunization (Table 1).
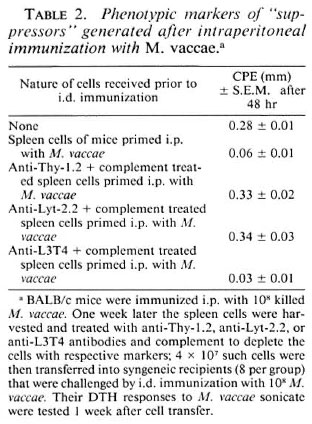
Phenotypic markers of "suppressors" generated after i.p. immunization with M. vaccae. In order to identify the cell type responsible for this suppression, BALB/c mice were given 108M. vaccae by the i.p. route as before. One week later their spleen cells were harvested and transferred to syngeneic mice (4 X 107 cells/mouse), that were then immunized intradermally (i.d.) with 108 M. vaccae. One week later, the DTH response to M. vaccae sonicate was tested and, as expected, suppression was observed. The ability of the immune spleen cells to adoptively transfer suppression could be completely abrogated by the removal of cells having Thy-1 and Lyt-2 markers by antiThy-1.2 and anti-Lyt-2.2 antibody and complement, indicating that the suppressors were, in fact, T-supprcssor cells (Ts) (Table 2).
Generation of Ts after i.p. immunization with M. tuberculosis H37Rv. Swiss white mice were given a subimmunogenic dose of M. tuberculosis H37Rv (105 bacilli/mouse) by the i.p. route and its effect on the immunogenicity of 108 bacilli given by the i.d. route was tested as before. Prior i.p. treatment with subimmunogenic doses of M. tuberculosis H37Rv suppressed generation of the DTH response by i.d. immunization (Table 1).
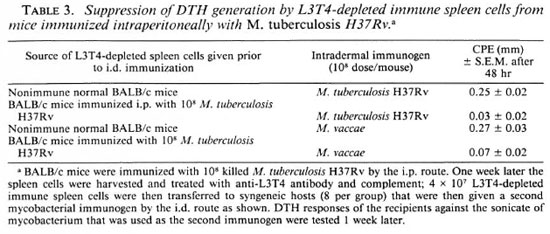
Since i.p. immunization with higher doses of M. tuberculosis H37Rv and other slow growers was earlier shown to generate DTH effectors (16), we had to employ a protocol consisting of adoptive syngeneic transfer after removal of DTH effectors. BALB/c mice were immunized i.p. with M. tuberculosis H37Rv (108 cells/mouse), and 1 week later their spleen cells were harvested. The cells were treated with anti-L3T4 antiserum and complement to remove DTH effectors, then adoptively transferred (4 X 107 cells/mouse) to syngeneic mice, which were then given an i.d. immunization of M. tuberculosis H37Rv (108 bacilli/mouse). One week later the DTH response to the sonicate of the immunizing organism was tested and total suppression was observed. Similar results were obtained when M. avium was used as an immunogen (Table 3).
Absence of T-suppressor response after i.d. immunization. Next, it was decided to investigate if i.d. immunization also generates a T-suppressor component in addition to a DTH response. For this, the same protocol of adoptive transfer after removal of DTH effectors was employed. BALB/c mice were immunized i.d. with 108 M. vaccae. Ok later their DTH response to M. vaccae sonicate was tested. All mice mounted an excellent DTH response, witne week later their spleen cells were harvested and treated with anti-L3T4 antiserum and complement to remove DTH effectors. The cells (4 X 107/mouse) were then transferred into syngeneic mice that were then challenged with an i.d. immunization of M. vaccae (108 bacilli/mouse). One weehout any evidence of suppression. Similar results were obtained with M. phlei and M. tuberculosis H37Rv as immunogens (Table 4).
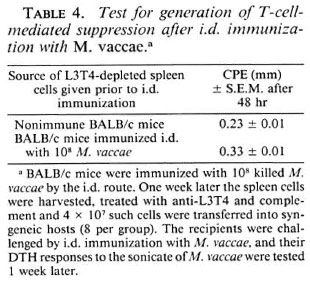
Based on these data, it was concluded that i.p. immunization with M. vaccae and other rapid growers predominantly generated T suppression. Intraperitoneal immunization with slow-growing mycobacteria generated both the DTH effector response (16) and the T-suppressor component. Intradermal immunization with both rapid and slow growers generated only a DTH effector response (16) no T-suppressor component could be shown.
Evidence of dose-dependent crosssuppression after i.p. immunization. Swiss white mice were immunized with M vaccae by the i.p. route employing two doses: 105and 108 bacilli/mouse. One week later the mice were given an i.d. immunization with M. vaccae, M. phlei, XI. tuberculosis H37Rv, or M. avium (108 bacilli/mouse). The DTH response to the sonicate of the second mycobacterium, employed as an immunogen by the i.d. route, was tested after 1 week, as before. Intraperitoneal immunization with M. vaccae completely abrogated generation of the DTH response by subsequent i.d. immunization with M. vaccae as before. However, suppression of the i.d. immunization by other mycobacteria was found to be dose dependent. At 105 bacilli/mouse, i.p. immunization with XI. vaccae suppressed subsequent i.d. immunization by rapid growers such as M. phlei only, and not by the slow growers. However, at higher doses, i.e., 108bacilli/mouse, i.p. immunization with M. vaccae suppressed DTH generation by i.d. immunization with all of the mycobacteria tested. It can be argued that the observed suppression is a nonspecific phenomenon. However, i.p. immunization with M. vaccae could not suppress subsequent immunization with Salmonella enteritidis, indicating that the suppression was not a nonspecific phenomenon (Table 1).
Evidence of dose-dependent crosssuppression was also observed with slow growers. Swiss white mice given XI. tuberculosis H37Rv by the i.p. route at a dose of 105 bacilli/mouse (subimmunogenic) suppressed subsequent i.d. immunization by other slow growers, such as XI. avium, but not by rapid growers, such as M. phlei and M. vaccae (Table 1).
As pointed out earlier, since higher doses of i.p. immunization with slow growers generated a DTH response, the same protocol that was employed for the demonstration of the generation of T suppression after an i.p. immunization was also employed for detection of cross-suppression. BALB/c mice were immunized with 108 M. tuberculosis H37Rv by the i.p. route. One week later their spleen cells were transferred into syngeneic recipients after depletion of L3T4-positivc cells, and the recipients were then immunized by the i.d. route with different mycobacteria (108 bacilli/mouse) to check for crosssuppression. It was observed that the DTH responses of the recipients to all of the mycobacteria, i.e., both slow and rapid growers, were suppressed (Table 3).
Thus, it was concluded that crosssuppression was observed after i.p. immunization with both slow and rapid growers. The degree of crosssuppression was dose dependent; at 105bacilli/mouse, rapid growers suppressed only rapid growers and vice versa and at higher doses, such as 108bacilli/mouse, immunization by all of the mycobacteria was suppressed.
In vitro evidence of generation of T suppressors following i.p. immunization with M. vaccae. BALB/c mice were immunized i.p. with 105and 108M. vaccae, with the protocols shown earlier to generate Tcellmediated suppression. The spleens of these mice were harvested, and single cell suspensions were made. A separate batch of BALB/c mice was immunized i.d. with 108 M. vaccae or M. tuberculosis H37Rv, a protocol shown earlier to generate a DTH effector response. The spleens of these mice were also harvested and made into a single cell suspension. Different numbers of (104to 5 × 105) spleen cells from mice primed i.p. with M. vaccae were cocultured with 2 × 105spleen cells from mice primed i.d. with M. vaccae or M. tuberculosis H37Rv. A standard 6 day lymphoproliferation test was performed in the presence of 10 μg/ culture ofsonicates ofthe respective organisms. It was observed (The Figure) that i.p. immunization with 105M. vaccae generated suppressors that could inhibit antigen-induced proliferation of the spleen cells from M. vaccae-primed mice. On the other hand, the suppressors generated by i.p. immunization with 108 M. vaccae could inhibit antigeninduced proliferation of not only M. vaccaeprimed spleen cells but also M. tuberculosis-primed spleen cells. The suppressive effects of i.p.-primed spleen cells could be completely abrogated by treatment with anti-Thy-1.2 and complement, indicating the suppression to be T-cell mediated. The in vitro experiments also showed the same pattern of doserelated crosssuppression as seen in the in vivo experiment.
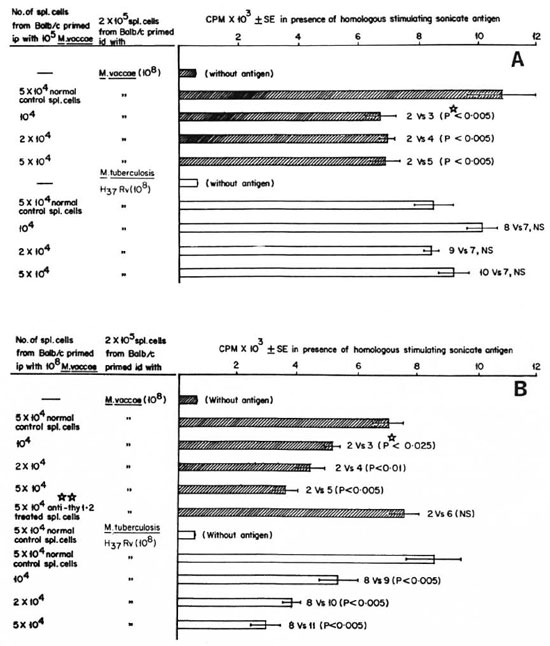
The Figure. In vitro suppression of antigen-induced proliferation by suppressor cells generated after i.p. immunization with M. vaccae: A = 105 cells/mouse; B = 108 cells/mouse. BALB/c mice were immunized id. with 108 M. vaccae or M. tuberculosis H37Rv and 1 week later spleen cells were harvested. Separate batches of BALB/c mice were primed i.p. with 105 or 108 M. vaccae, and the spleen cells harvested 1 week later: 2 x 105 spleen cells of id-primed mice were mixed with different numbers of spleen cells of i.p.-primed or normal mice and a standard 6-day lymphoproliferative test was performed with 10 μg/m1 of M. vaccae or M. tuberculosis H37Rv sonicate, respectively.  = proliferation of M. vaccae-primed spleen cells;
= proliferation of M. vaccae-primed spleen cells;  = proliferation of M. tuberculosis-primed spleen cells; CPM = mean count per minute of 5 wells ± S.E.M. (standard error of the mean); p
= proliferation of M. tuberculosis-primed spleen cells; CPM = mean count per minute of 5 wells ± S.E.M. (standard error of the mean); p = Student's t test; NS = not significant;
= Student's t test; NS = not significant; 
 = spleen cells of BALB/c mice primed i.p. with 108 M. vaccae were treated with anti-Thy-1.2 and complement to kill T cells and the suppressive potential of these cells was tested.
= spleen cells of BALB/c mice primed i.p. with 108 M. vaccae were treated with anti-Thy-1.2 and complement to kill T cells and the suppressive potential of these cells was tested.
DISCUSSION
Variation in immunogenicity when antigen is introduced by different routes has been known for some time. Based on the responses of mice to i.p. and i.d. immunization with M. leprae, Shepard, et al. (13) have proposed that the observed suppression in lepromatous leprosy could be related to the route of infection. Reports in the literature offer evidence of immune suppression in leprosy(9,10),although the generation ofthe classical CD8positive antigenspecific Tsuppressor cell (Ts) has not been universally accepted. Further, both leprosy and tuberculosis are disorders in which organisms are introduced into the body by different routes. However, the usual form of clinical tuberculosis is not associated with pronounced immune suppression as seen in lepromatous leprosy. Hence, it is important to learn about the factors that lead to generation of specific immune suppression on exposure to antigen.
Employing the experimental model of study ofimmunogenicity by different routes described by Shepard, et al. (13), a number ofcultivable mycobacteria were investigated for their ability to generate suppression, and the phenomenon was further investigated to determine the phenotype ofthe cells causing suppression, the antigen specificity of the suppression, and if the observed suppression was a minor or a predominant component ofthe immune response. Essentially, the model investigates generation of Ts when antigen is presented by Langerhans'cellsand peritoneal macrophages. The former are rich in MHC class II or Ia antigens, and have been shown to generate a good Tcell response with practically all mycobacteria. In contrast, peritoneal macrophages are poor in Ia composition and also poor in their antigenpresenting potential, particularly for M. vaccae (15). Several cell types in the body, such as endothelial cells, tissue macrophages, etc., express variable amounts of Ia (8) and, as a result, the data generated here have parallel clinical implications.
For a study ofimmune suppression at the cellular level, it was essential to employ adoptive transfer techniques which could not be undertaken in the outbred Swiss white strain of mice. Hence, all such experiments were carried out in the inbred BALB/c strains. Prior to undertaking such studies, it was confirmed that the immunogenicity and suppressor responses of BALB/c mice to different mycobacteria were identical to those seen in Swiss white mice.
It is interesting to note that in i.p. immunization with M. tuberculosis H37Rv and other slow growers, generation of the Ts response was only a component; whereas with M. vaccae and the other rapid growers the Ts response was the predominant response after i.p. immunization. Here it may be noted that naive normal peritoneal cells could present M. tuberculosis H37Rv antigen efficiently in an in vitro lymphoproliferative test; in contrast, presentation of M. vaccae antigen by peritoneal cells was poor (15). Intradermal immunization failed to generate a Ts component, even with M. vaccae. Incidentally, all of the mycobacteria tested, including M. vaccae and the other rapid growers, were immunogenic by the i.d. route. Thus the data clearly show not only differences in the immunogenicity by the two routes of immunization, they also show differences in the ability to generate Ts when antigen is introduced by the two routes.
Poor presentation of M. vaccae by peritoneal cells has been ascribed to the low density of MHC class II or Ia antigens on them. Differences in the presentation efficiency of M. vaccae and M. tuberculosis H37Rv by peritoneal cells was thought to be the result of the inability of M. vaccae antigens to form a stable association with Ia in the peritoneal macrophage membrane, thus implicating the Ir genes (15). Selective generation of Ts against M. vaccae by i.p. immunization and its absence on i.d. immunization could also be a phenomenon dependent upon the differential Ia composition of the two antigen-presenting cell types involved, consequently resulting in differences in their antigen-presenting potential. The hypothesis thus implicates the so-called immune suppression (Is) genes (2). Distinct differences in the MHC restriction of T-helper and T-suppressor cell recognition have been reported, even against infectious and parasitic agents (1).
It is noteworthy that both rapid and slow growers generated Ts that showed extensive crossreactivity, although the spectrum was dose dependent. The phenomenon is interesting because subclinical infection with environmental mycobacteria has been shown to adversely modify the ability of the host to respond to BCG vaccination (14).
The data reported here are also significant in another context. The immunogenicity of M. leprae is very similar to that of M. vaccae-it is immunogenic by the i.d. route only and, further, prior i.p. immunization suppresses subsequent i.d. immunization (13). M. vaccae has also been reported to be immunologically related to M. leprae(17, 20).
Acknowledgment. K. E. Shroff thanks the University Grants Commission, Government of India, for a fellowship grant to undertake this study.
REFERENCES
1. BAXEVANIX. C. N., NAGY, Z. A. and KLEIN, J. A novel type of T-T cell interaction removes the requirement of I-B region in the H-2 complex. Proc. Natl. Acad. Sci. U.S.A. 78(1981)3809-3813.
2. DEBRÉ, P., KAPP, J. A., DORF, M. E. and BENA-CERAF, B. Genetic control of specific immune suppression. II. H-2-linked dominant genetic control of immune suppression by the random copolymer L-glutamic acid50-L-tyrosine50 (GT). J. Exp. Med. 142(1975)1447-1454.
3. DOUB, L. and YOUMANS, G. P. Studies in tuberculosis chemotherapy; simply primary aromatic amines, in vitro and in vivo. Am. Rev. Tuberc. 61(1950)407-421.
4. GEORGE, A., RATH, S. and KAMAT, R. S. Differential regulation of effector reponses of cell mediated immunity in experimental salmonellosis. Clin. Exp. Immunol. 63(1986)327-333.
5. GRAY, D. F. and JENNINGS, P. A. Allergy in experimental tuberculosis. Am. Rev. Respir. Dis. 72(1955)171-195.
6. LENZINI, L., ROTTOLI, P. and ROTTOLI, L. The spectrum of human tuberculosis. Clin. Exp. Immunol. 27(1977)230-237.
7. LOWRY, O. H., ROSEBROUGH, N. J., FARR, A. L. and RANDALL, R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193(1951)265-275.
8. MASON, D. W. and BARCLAY, A. N. Constitutive and inducible expression of la antigens. Immunobiology 168(1984)167-171.
9. MEHRA, V., CONVIT, J., RUBINSTEIN, A. and BLOOM, B. R. Activated suppressor T cells in leprosy. J. Immunol. 129(1982)1946-1951.
10. Nath, I. and SINGH, R. Suppressive effects of M. leprae on the in vitro proliferative response of lymphocytes from patients with leprosy. Clin. Exp. Immunol. 41(1980)406-414.
11. OPPENHEIM, J. J. and SCHECHTER, B. Lymphocyte transformation. In: Manual of Clinical Immunology. Rose, N. R. and Friedman, H., eds. Washington, D.C.: American Society for Microbiology, 1976, pp. 81-94.
12. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)251-273.
13. SHEPARD, C. C, WALKER, L., VAN LANDINGHAM, R. M. and YE, S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of infection. Infect. Immun. 38(1982)673-680.
14. SHIELD, M. J., STANFORD, J. L. and ROOK, G. A. W. The reason for the reduction of protective efficacy of BCG in Burma. Abstract in Int. J. Lepr. 47(1979)319-320.
15. SHROFF, K. E., SAINIS, K. B., SENGUPTA, S. R. and KAMAT, R. S. Variation in immunogenicity of mycobacteria: role of antigen-presenting cells. Int. J. Lepr. 58(1990)58-64.
16. SHROFF, K. E., SENGUPTA, S. R. and KAMAT, R. S. Route-related variation in immunogenicity of mycobacteria. Int. J. Lepr. 58(1990)44-49.
17. STANFORD, J. L., ROOK, G. A. W., CONVIT, J., GODAL, T., KRONVALL, G., REES, R. J. W. and WALSH, G. P. Preliminary taxonomic studies on the leprosy bacillus. Br. J. Exp. Pathol. 56(1975)579-585.
18. VAN EDEN, W. and DE VRIES, R. R. P. Occasional review -HLA and leprosy: a re-evaluation. Lepr. Rev. 55(1984)89-104.
19. VAN EDEN, W., ELFERINK, B. G., DE VRIES, R. R. P., LEIKER, D. L. and VAN ROOD, J. J. Low T-lymphocyte responsiveness to M. leprae antigen in association with HLA-DR3. Clin. Exp. Immunol. 55(1984)140-148.
20. WATSON, S. R., MORRISON, N. E. and COLLINS, F. M. Delayed hypersensitivity response in mice and guinea pigs to Mycobacterium leprae, Mycobacterium vaccae, and Mycobacterium nonchromogenicum cytoplasmic proteins. Infect. Immun. 25(1979)229-236.
1. K. E. Shroff, M.Sc, Research Fellow, Department of Immunology, Haffkine Institute, Parel, Bombay 400012, India.
2. S. R. Sengupta, M.D., Director, Department of Immunology, Haffkine Institute, Parel, Bombay 400012, India.
3. R. S. Kamat, M.D., Assistant Director and Head, Department of Immunology, Haffkine Institute, Parel, Bombay 400012, India.
Reprint requests to Dr. Kamat.
Received for publication on 5 August 1988.
Accepted for publication in revised form on 17 August 1989.