- Volume 58 , Number 1
- Page: 58–64
Variation in immunogenicity of mycobacteria: role of antigen-presenting cells
ABSTRACT
The antigen-presenting efficiency of peritoneal cells and irradiated spleen cells was compared using Mycobacterium tuberculosis- and M. vaccae-primed T cells and corresponding sonicates as antigens in an in vitro lymphocyte transformation test. The presentation efficiency of irradiated spleen cells was reasonably good for both antigens. However, with peritoneal cells as the antigen-presenting cells, the proliferative response against only M. tuberculosis sonicate was good. Proliferation of M. vaccae-primed T cells was very poor when the antigen was presented by peritoneal cells. Poly I: poly C treatment of mice prior to harvesting the peritoneal cells resulted in distinct improvement in their efficiency to present M. vaccae sonicate; maximal proliferative response was obtained with peritoneal cells from mice receiving two and three doses of poly I: poly C 24 hr apart. Even paraformaldehyde-fixed peritoneal cells from poly I: poly C-treated mice gave an efficient M. vaccae-specific stimulation to primed T cells. Based on these data, it was concluded that failure of mice to respond to M. vaccae by intraperitoneal immunization is the result of the poor efficiency of presentation of M. vaccae antigen.
RÉSUMÉ
On a comparé l'efficacité de la présentation des antigènes par les cellules péritéonéales et par les cellules de rate irradiée, en utilisant des cellules-T sensibilisées à Mycobacterium tuberculosis et à M. vaccae ainsi que des sonicats correspondants, comme antigènes dans une épreuve de transformation lymphocitaire in vitro. Avec l'un et l'autre de ces antigènes, les cellules splecniques irradiées ont assuré cette présentation avec une efficacité raisonnable. Néanmoins, lorsqu'on utilisait des cellules péritéonéales pour présenter les antigènes, seule une réponse proliferative contre le sonicat de M. tuberculosis s'est révélée satisfaisante. La prolifération des cellules-T sensibilisées à M. vaccae était très faible lorsque l'antigène était présenté par les cellules péritéonéales. Un traitement préalable des souris par le poly I : poly C, avant la récolte des cellules péritéonéales, a signilicativement augmenté l'efficacité de ces cellules pour la présentation des antigènes avec les sonicats de M. vaccae. Une réponse proliferative maximale a été obtenue lorsqu'on utilisait des cellules péritéonéales de souris ayant reçu deux et trois doses de poly I : poly C à 24 heures d'intervalle. On a observé que les cellules péritéonéales de souris traitées par le poly I : poly C témoignaient d'une stimulation aux cellules-T sensibilisées spécifiquement à M. vaccae. même lorsqu'elles étaient fixées par la paraformaldehyde. Sur base de ces données on conclut que l'incapacité des souris à répondre à M. vaccae lors d'une immunisation intrapéritéonéale est la conséquence d'une déficience dans la présentation de l'antigène M. vaccae.
RESUMEN
Mediante un ensayo de proliferación in vitro de linfocitos se comparó la eficiencia presentadora de antígeno de las células peritoneales y de las células irradiadas del bazo de ratón. Se usaron células T preestimuladas con Mycobacterium tuberculosis y M. vaccae y los correspondientes antigenos sonicados. La eficiencia presentadora de antígeno de las células irradiadas del bazo fue razonablemente buena con ambos antígenos. Sin embargo, con las células peritoneales como presentadoras de antigeno, sólo fue buena la respuesta contra el sonicado de M. tuberculosis. La proliferación de células T preestimuladas con M. vaccae fue muy pobre cuando el antígeno fue presentado por las células peritoneales. El tratamiento de los ratones con poli I: poli C antes de cosechar las células peritoneales, dió como resultado una notable mejoría en su eficiencia presentadora de antígeno de M. vaccae; la máxima respuesta proliferativa se obtuvo con células peritoneales de animales que recibieron 2 y 3 dosis de poli I: poli C, una cada 24 horas. Aún las células peritoneales fijadas con paraformaldehído de los animales tratados con poli I: poli C, estimularon eficientemente a las células T preestimuladas con M. vaccae. Con baso en estos datos se concluyó que la falla de los ratones para responder al M. vaccae por la inmunización intraperitoneal es el resultado de Ia pobre eficiencia en la presentación de antígeno de M. vaccae.
The pathogenesis of the immunological nonresponder state observed in mycobacterial infections (8, 11) is still far from clear. Some investigators have suggested a genetic defect in antigen presentation to be the likely cause of the nonrespondcr state (15, 16). Based on the outcome of immunization by different routes, others have disputed the role of antigen presentation in the pathogenesis of nonrespondcr states (12) In an accompanying publication (14), we present evidence to suggest that the routerelated variation in the immunogenicity of Mycobacterium vaccae, an organism antigenically related to M. leprae (17), is likely due to variation in the presentation efficiency of antigenpresenting cells (APCs) encountering the antigen. In this publication, the efficiency of different APCs to present mycobacterial antigens in a lymphoprolifcration test is compared before and after modulation with agents known to enhance expression of major histocompatibility complex (MHC) glycoproteins that are so important in antigen presentation.
MATERIALS AND METHODS
M. tuberculosis H37Rv (NCTC 7416) was purchased from the National Collection of Type Cultures, London. M. vaccae was kindly supplied by Dr. J. L. Stanford of Middlesex Hospital, London.
BALB/c mice, 46 weeks old, were obtained from the National Institutes of Health, Bethesda, Maryland, U.S.A., and maintained at the Cancer Research Institute, Bombay, India, until used in the study.
Preparation of sonic extracts of the organisms, immunization with mycobacterial cells by the intraperitoneal (i.p.) and intradermal (i.d.) routes, and dclaycdtype hypersensitivity (DTH) tests were carried out using techniques already described (13).
Lymphoproliferation test. A lymphoproliferation test was carried out employing standard microculture technique (10). Briefly, each well of a microtitcr plate (Nunc, Denmark) received 2 X 105 immune T cells alongwith2 X 104 (10%) or 5 X 104 (25%) different syngeneic nonimmune cells (as APCs) in 0.2 ml of culture medium RPMI 1640 containing 2.0 mM glutamine, 10% fetal calf serum (FCS), 1% antibiotic antimycotic (AA; GIBCO, Grand Island, New York, U.S.A.) and 10 μg/ml of sonicate of the mycobacterial culture under test. Cultures were pulsed 120 hr later with 0.5 μCi of 3Hlabeled thymidine (BARC, India; sp. act. 2 Ci/mMol) for a period of 16 hr, harvested, and the incorporation of the label in cellular DNA assayed. All tests were run in quintuplicate. Whenever required, the source of the APCs was subjected to 1200 rad irradiation in a Co60 source to eliminate T-and B-cell responses to antigen.
Immune T cells. BALB/c mice were immunized i.d. with 108killed cells of either M. vaccae or M. tuberculosis H37Rv. One week later successful immunization was confirmed by tests for DTH. The mice were then sacrificed by cervical dislocation and their spleens were harvested. Single cell suspensions were made in the wash medium, Hanks' balanced salt solution (HBSS) containing 10% FCS and 1% AA. Cells of several mice were pooled and suspended in HBSS containing 0.83% ammonium chloride to lyse the red cells, washed three times, and the viability estimated. The cells were then loaded onto a nylon wool column and nonadherent cells eluted as described by Julius, et al. (6). The nonadherent cells were washed once, suspended in the culture medium, and the viability estimated.
Peritoneal cells. Peritoneal cells were collected from normal BALB/c mice and those treated intravenously (i.v.) with poly I: poly C. The mice were killed and within 5 min an i.p. injection of 4.0 ml wash medium was given. Their abdomens were massaged gently and the contents were aspirated by a sterile syringe. Aspirates ofseveral mice were pooled, the cells washed three times, and the viable cell number estimated.
Antigen pulsing of peritoneal cells. Peritoneal cells (4 X 105) were suspended in 2.0 ml of the culture medium containing 200 μg of the sonicate of M. tuberculosis H37Rv or M. vaccae and incubated at 30ºC for 60 min. They were then washed three times, and the final pellet was suspended in the culture medium.
Fixation. Paraformaldehyde fixation of peritoneal cells was done by the method described by Allen and Unanue (1). Briefly, antigenpulsed or nonpulsed 4 X 105 peritoneal cells were suspended in 2.0 ml HBSS containing 1% (w/v) paraformaldehyde (analytical grade; BDH, Ltd., Poole, U.K.) and incubated at 20ºC for 15 min. At the end of the incubation period, they were washed three times, incubated again at 37ºC in the culture medium, and again washed three times.
Enumeration of MHC class II or Iaexpressing cells. Peritoneal and spleen cells of BALB/c mice were stained for MHC class II or la antigens employing the indirect immunofluorescence technique (5). Cells (106-107) were suspended in 0.1 ml of rat monoclonal antibody of I-Ab,d, q I-Ed,k specificity and kept on ice for 30 min. The cells were washed three times and suspended in 50 μl of commercial FITC coupled rabbit antirat globulin (Miles-Yeda, Israel). They were incubated on ice for 30 min. The suspensions were washed and the pellets were suspended in a drop of FCS. Smears were prepared and examined on a fluorescent microscope.
Antibody to MHC class II. The supernates of cultures of rat IgG2b clone of antibody specificity I-Ab,d,q IEd,k (4) were pooled. The antibody was precipitated with 33% (NH4)2S04, dissolved in PBS, and dialyzed extensively. For staining, the protein concentration was adjusted to 500 μg/ml.
RESULTS
In vitro lymphoproliferation test to compare presentation efficiency of peritoneal cells and splenic APCs. BALB/c mice were immunized with 108 killed M. vaccae or M. tuberculosis H37Rv by the i.d. route. Their spleen cells were harvested 1 week later, and the immune T cells were separated.
Primed T cells (2 × 105) were cocultured with different syngeneic nonimmune cells as APCs to compare the efficiency of different APCs to present M. vaccae and M. tuberculosis H37Rv antigens. Two concentrations of APCs, namely, 2 × 104(100%) and 5 × 104(25%), were employed. A standard 6-day lymphoproliferation test was performed with 10 μg/culture of sonicate of M. vaccae or M. tuberculosis H37Rv as antigens and immune T cells from mice primed with the respective bacterial vaccine.
With irradiated spleen cells as APCs, the M. tuberculosis primed T cells gave a good antigeninduced proliferative response against the sonicate of the organism. With peritoneal cells from nonimmune naive mice as APCs, the proliferative response of M. tuberculosis-primed T cells was also good; at the 25% peritoneal cell concentration, the response was of the same order as that seen with spleen cells (Fig. 1). The pattern of responses of M. vaccae-primed T cells was quite different. As with M. tuberculosis-primed T cells, the M. vaccae-primed T cells also showed a good proliferative response against the sonicate of the organism with irradiated spleen cells as APCs. However, with peritoneal cells from nonimmune naive mice as APCs, the proliferative response was very poor at both concentrations of APCs. When peritoneal cells from poly I: poly Ctreated mice were used as APCs, the proliferative response of M. vaccae-primed T cells showed a distinct improvement and reached the same order as that seen with irradiated spleen cells as APCs (Fig. 2).
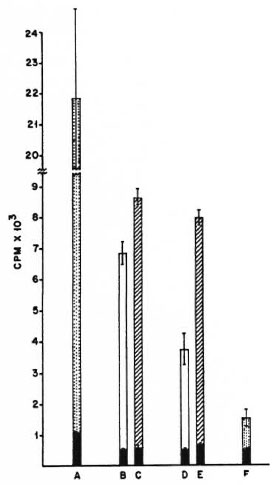
Fig. 1. Proliferation of 2 × 105 M. tuberculosis H37Rv-primed, splenic T cells co-cultured with different antigen-presenting cells and 10 μg/culture of the sonicate of the organism as antigen. A = Proliferation of the whole unfractionated primed spleen cells; B and C = irradiated normal spleen cells as APCs; D and E= irradiated peritoneal cells from nonimmune normalmice as APCs; F = proliferation of normal, nonim-mune, whole unfractionated spleen cells; CPM = meancounts per min of 5 culture wells ± S.E.M. (as bars);  = no antigen;
= no antigen;  = 2 × 104 APCs and sonicate antigen;
= 2 × 104 APCs and sonicate antigen;  = 5 × 104 APCs and sonicate antigen;
= 5 × 104 APCs and sonicate antigen;  = wholeunfractionated spleen cells and sonicate antigen.
= wholeunfractionated spleen cells and sonicate antigen.
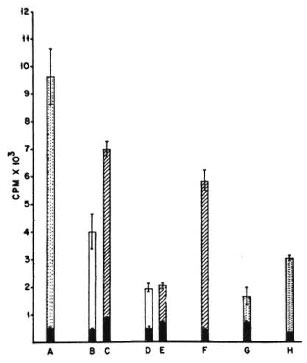
Fig. 2. Proliferation of 2 x 105 M. vaccae-primed(i.d.) splenic T cells co-cultured with different APCs and 10 μg/culture of the sonicate of the organism as antigen. A = proliferation of the whole unfractionated primed spleen cells; B and C = irradiated normal spleen cells as APCs; D and E = irradiated peritoneal cells from nonimmune normal mice as APCs; F = irradiated peritoneal cells from poly I: poly C-treated mice as APCs; G = proliferation of normal, unfractionated, whole spleen cells, H = proliferation of unfractionated whole spleen cells primed i.p. with M. vaccae; CPM = mean counts per min of 5 culture wells ± S.E.M. (asbars);  = no antigen;
= no antigen;  = 2 × 104 APCs and sonicate antigen;
= 2 × 104 APCs and sonicate antigen;  = 5 × 104 APCs and sonicate antigen;
= 5 × 104 APCs and sonicate antigen;  = whole unfractionated spleen cells and sonicate antigen.
= whole unfractionated spleen cells and sonicate antigen.
From these data it can be concluded that the presentation efficiency of irradiated spleen cells was similar and reasonably good for both antigens, namely, M. vaccae and M. tuberculosis sonicates. However, with peritoneal cells as APCs, although presentation of M. tuberculosis antigens was good, M. vaccae presentation was poor. Further treatment with poly I: poly C brought about a distinct improvement in the presentation of M. vaccae by peritoneal cells.
Effect of dose and duration of poly I: poly C treatment on efficiency of presentation of M. vaccae. BALB/c mice were given one, two, three, and five i.v. injections of 100 μg of poly I: poly C at 24hr intervals, and their peritoneal cells were harvested 3 hr after the last injection. As before, 2 × 105 M. vaccae-primed splenic T cells were cocultured with 10% and 25% peritoneal cells so harvested and a lymphoproliferative test was performed. Poly I: poly C treatment of mice resulted in a distinct improvement in presentation efficiency and maximal stimulation was given by peritoneal cells of mice receiving two and three doses. Further treatment with five doses did not result in any greater stimulation (Fig. 3).
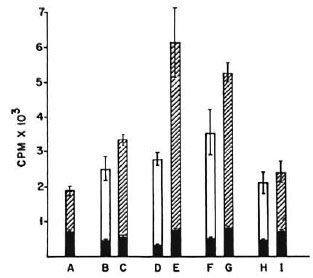
Fig. 3. Proliferation of 2 × 10 M. vaccae-primed splenic T cells co-cultured with peritoneal cells from nonimmune normal mice (A) or mice treated with one (B, C), two (D, E), three (F, G), and five (H, I) doses of poly I: poly C at 24-hr intervals. CPM = mean counts per min of 5 culture wells ± S.E.M. (as bars);  = no antigen;
= no antigen;  = 2 × 104 peritoneal cells and M. vaccae sonicate antigen;
= 2 × 104 peritoneal cells and M. vaccae sonicate antigen;  = 5 × 104 peritoneal cells and M. vaccae sonicate antigen.
= 5 × 104 peritoneal cells and M. vaccae sonicate antigen.
Presentation of M. vaccae antigen by paraformaldehydefixed peritoneal cells from naive and poly I: poly Ctreated mice. Peritoneal cells from normal naive BALB/c mice and those treated with different doses of poly I: poly C were harvested as before and pulsed with M. vaccae sonicate. These cells were then used as APCs in a lymphoproliferative test, either directly or after paraformaldehyde fixation. As before, when fixed or unfixed peritoneal cells from naive animals were used in the assay, the proliferative response was very poor. However, when peritoneal cells from poly I: poly C-treated mice were used in the assay, a good proliferative response was observed, even after paraformaldehyde fixation. As before, maximal proliferation was seen with peritoneal cells from mice treated with two and three doses of poly I: poly C (Fig. 4).
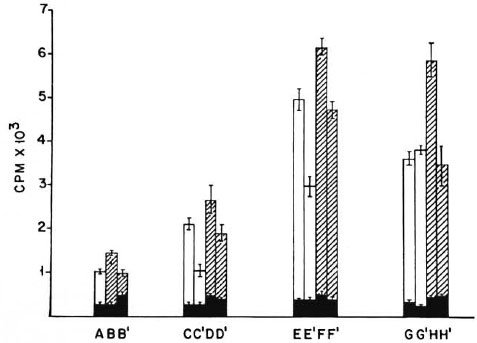
Fig. 4. Proliferation of 2 x 105 M. vaccae-primed T cells co-cultured with M. vaccae sonicate pulsed and paraformaldehyde fixed or unfixed peritoneal cells as APCs. A, B, B' = peritoneal cells from normal nonimmunemice; C, C', D, D' = peritoneal cells from mice receiving one dose of poly I: poly C; E, E', F, F' = peritoneal cells from mice receiving two doses of poly I: poly C 24 hr apart; G, G', H, H' = peritoneal cells from micereceiving three doses of poly I: poly C 24 hr apart; CPM = mean counts per min of 5 culture wells ± S.E.M.(as bars);  = peritoneal cells not pulsed with antigen;
= peritoneal cells not pulsed with antigen;  = 2 x 104 peritoneal cells pulsed with M. vaccae sonicate;
= 2 x 104 peritoneal cells pulsed with M. vaccae sonicate;  = 5 x 104 peritoneal cells pulsed with M. vaccae sonicate; B', C', D', E', F', G', and H' cultures contain paraformaldehyde-fixed peritoneal cells.
= 5 x 104 peritoneal cells pulsed with M. vaccae sonicate; B', C', D', E', F', G', and H' cultures contain paraformaldehyde-fixed peritoneal cells.
Ia expression by peritoneal and spleen cells. Peritoneal and spleen cells harvested from normal and poly I: poly C-treated mice were stained for Ia antigens. Only 5.95% of the peritoneal cells were Ia positive; whereas 38.3% of the spleen cells were Ia positive. Poly I: poly C treatment resulted in a rapid increase in the la-positive peritoneal cells, rising from 5.95% to 18% in just 3 hr. Treatment with two and three doses of poly I: poly C did not result in any further significant rise in the percentage of la-positive peritoneal cells, it being 24.5% after three doses.
DISCUSSION
The superiority of the intradermal (i.d.) route over the intraperitoneal (i.p.) route of immunization to generate cell-mediated immunity (CMI) against M. leprae is well known (12). In addition, we could show that i.p. immunization generates suppression (13). However, failure to generate cellular immunity by the i.p. route is not a universal phenomenon, even for the genus Mycobacterium as has been alleged (12). Despite the generation of T-suppressor (Ts) cells upon i.p. immunization (13), the slow growers behaved as good immunogens by this route, as judged by the delayed-type hypersensitivity (DTH) and lymphoproliferative responses (14). The differences in the immunogenicity of slow and rapid growers by the i.p. route of immunization, despite the generation of Ts cells by both groups, indicate that generation of Ts cells alone cannot explain the nonimmunogenicity of rapid growers by the i.p. route. It was possible to correct the nonimmunogenicity of rapid growers by prior treatment of mice with agents that enhance Ia expression, such as poly I: poly C, and interferon (INF) inducer, and indomethacin, a prostaglandin inhibitor (14). In light of this evidence, it was felt that the ability of peritoneal cells to present the rapid- and slow-grower antigens might play a crucial role in the immunogenicity of the two groups by the i.p. route of immunization.
Hence, it was decided to study and compare the efficiency of presentation of M. vaccae and M. tuberculosis antigens by peritoneal cells and irradiated spleen cells to the respective antigen-primed T cells in an in vitro lymphoproliferative response. Since spleen cells contain dendritic cells which are potent antigen-presenting cells, irradiated spleen cells were employed as the reference source of good antigen presenters. Since i.d. immunization triggered a T-cell response to both M. vaccae and M. tuberculosis H37Rv (14), splenic T cells of i.d.-primed mice were employed as antigen-primed T cells.
The peritoneal cell-induced T-cell stimulation against M. tuberculosis H37Rv was as efficient as that induced by the irradiated spleen cells. However, with M. vaccae sonicate only the irradiated spleen cells could provide adequate antigenic stimulation to the primed T cells; the stimulation induced by peritoneal cells was extremely poor. The latter showed distinct improvement in their ability to provide stimulation with M. vaccae antigen, even within 3 hours after a single dose of the IFN inducer poly I: poly C was given to mice prior to harvesting the peritoneal cells. A peak response to poly I: poly C was seen after two and three doses given at 24-hour intervals; any further treatment did not result in further improvement.
To confirm that these effects of poly I: poly C treatment were due to improvement in antigen presentation only, the above experiments were repeated with antigen-pulsed paraformaldehyde-fixed peritoneal cells. As expected, even the fixed peritoneal cells could provide adequate antigenic stimulation (with M. vaccae sonicate) only when they were obtained from poly I : poly C-treated mice.
Thus, both factors, namely, the efficiency of presentation and the generation of Ts cells, seem to decide the outcome of immunization by the i.p. route. When presentation is poor, as with rapid growers, Ts cells dominate, resulting in failure to generate the immune response. When presentation is efficient, despite generation of the Ts cells, the animals successfully mount a CMI response, as was observed with slow growers.
A clear cut elucidation of the role of antigen presentation in the pathogenesis of nonresponder states in mycobacterial infections is necessary. A MHC (DR)-linked presentation defect in lepromatous leprosy (LL) has been reported (15, 16), implicating the particular MHC class II allelic glycoproteins to be responsible for the defective presentation. This has been disputed by others on the grounds that the same outbred or inbred animal behaves as a responder by the i.d. route and a nonresponder by the i.p. route of immunization with M. leprae, hence, it could not be a genetic defect (12). Because of MHC restrictions imposed on T-cell recognition, two factors would govern the presentation efficiency of a given APC. Firstly, it is essential that the antigen forms a stable association with the polymorphous Ia glycoproteins in the membrane of the APC (2). Secondly, the APC processing the antigen has to express the Ia antigens in a requisite amount. In fact, employing defined antigenic systems, Matis, et al. (9) have clearly demonstrated the magnitude of the T-cell response to be a function of both the concentration of antigen as well as Ia glycoproteins. If one analyzes the present data in line with these arguments, the following conclusions pertaining to immunogenicity of mycobacteria emerge.
The presentation of M. vaccae by peritoneal cells was poor and their Ia density was low. Poly I: poly C treatment resulted in enhancement of the Ia expression by them along with improvement in their efficiency of M. vaccae presentation. It could therefore be postulated that antigens which form a stable association with Ia get presented adequately even with the APCs having low Ia density, as in the case of M. tuberculosis H37Rv; hence, the organism is immunogenic by both i.p. and i.d. routes. In contrast, the antigens that associate with Ia with marginal efficiency get presented adequately by the APCs rich in Ia, such as Langcrhans' cells and dendritic cells, and poorly by APCs that arc poor in Ia composition, such as peritoneal cells (3, 7). Further, enhancement of Ia expression in such la-deficient APCs would result in improvement in the presentation of such antigens. Thus, the selective in vivo and in vitro nonresponder state of mice to M. vaccae and other rapid growers when their antigens are presented by peritoneal cells, and the reversal of this defect by prior treatment with an IFN inducer, can also be considered as an expression of a MHC-restricted phenomenon. Several cell types which have the potential of antigen presentation but lack adequate density of MHC class II glycoproteins, such as capillary endothelium, tissue macrophages, alveolar lining cells, etc., might behave like peritoneal cells, and thus may play an important role in the immunopathology of mycobacterial infections.
Acknowledgment. K. E. Shroff thanks the University Grants Commission, Government of India, for a fellowship grant to undertake this study.
REFERENCES
1. ALLEN, P. M. and UNANUE, E. R. Differential requirements for antigen processing by macrophage for lysozyme specific T cell hybridomas. J. Immunol. 132(1984)1077-1079.
2. BAUBITT, B., ALLEN, P. M., MATSUEDA, G., HABER, E. and UNANUE, E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature 317(1985)359-361.
3. BELLER, D. I., KIELY, J. M. and UNANUE, E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunological stimuli. J. Immunol. 124(1980)1426-1432.
4. BHATTACHARYA. A., DORE. M. E. and SPRINGER, T. A. A shared alloantigenic determinant on Ia antigens encoded by I-A and I-E subregions: evidence for I region gene duplication. J. Immunol. 127(1981)2488-2495.
5. HUDSON, L. and HAY. F. C. Immunofluorescent staining of lymphocyte membranes. In: Practical Immunology. London: Blackwell Scientific Publ., 1980, pp. 33-35.
6. JULIUS, M. H.. SIMPSON, E. and HERZENBERG, L. A. A rapid method for the isolation of functional thymus derived murine lymphocytes. Eur. J. Immunol. 3(1973)645-650.
7. KATZ, D. R. Differences in accessory cell functions. Imnumobiology 168 (1984) 134-140.
8. LENZINI. L, ROTTOLI, P. and ROTTOLI, L. The spectrum of human tuberculosis. Clin. Exp. Immunol. 27(1977)230-237.
9. MATIS. L. A.. GLINCHER, L. H., PAUL, W. E. and SCHWARTZ. R. H. Magnitude of response of HLA restricted T cell clones is a function of the product of concentration of antigen and la molecules. Proc. Natl. Acad. Sci. U.S.A. 80(1983)6019-6022.
10. OPPENHEIM, J. J. and SCHECHTER, B. Lymphocyte transformation. In: Manual of Clinical Immunology. Rose, N. R. and Friedman. H.. eds. Washington, D.C.: American Society for Microbiology, 1976, pp. 81-94.
11. RIDLEY. D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34(1966)251-273.
12. SHEPARD, C. C, WALTER. L., VAN LANDINGHAM, R. M. and YE, S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of infection. Infect. Immun. 38(1982)673-680.
13. SCHROFF, K. E., SENGUPTA, S. R. and KAMAT, R. S. Pathogenesis of route-related variation in T-suppressor response on immunization with mycobacteria. Int. J. Lepr. 58 (1990) 50-57.
14. SCHROFF, K. E.. SENGUPTA, S. R. and KAMAT. R. S. Route-related variation in immunogenicity of mycobacteria. Int. J. Lepr. 58(1990)44-49.
15. VAN EDEN. W. and DE VRIES, R. R. P. Occasional review-HLA and leprosy: a re-evaluation. Lepr. Rev. 55(1984)89-104.
16. VAN EDEN, W., ELFERINK. B. G., DE VRIES, R. R. P.. LEIKER. D. L. and VAN ROOD, J. J. Low T-lymphocyte responsiveness to M. leprae antigen in association with HLA-DR3. Clin. Exp. Immunol. 55(1984)140-148.
17. WATSON, S. R., MORRISON, N. E. and COLLINS, F. M. Delayed hypersensitivity response in mice and guinea pigs to Mycobacterium leprae, Mycobacterium vaccae, and Mycobacterium nonchromogenicum cytoplasmic proteins. Infect. Immun. 25(1979)229-236.
1. M.Sc, Research Fellow, Department of Immunology, Haffkine Institute, Parel, Bombay 400012, India.
2. M.D., Director, Department of Immunology, Haffkine Institute, Parel, Bombay 400012, India.
3. M.D., Assistant Director and Head, Department of Immunology, Haffkine Institute, Parel, Bombay 400012, India.
4. Ph.D., Scientific Officer (SOSS), Molecular Biology and Agriculture Division, Biology Group, Bhabha Atomic Research Centre, Trombay, Bombay, India.
Reprint requests to Dr. Kamat.
Received for publication on 5 August 1988.
Accepted for publication in revised form on 17 August 1989.