- Volume 58 , Number 1
- Page: 65–72
Antibodies to lipoarabinomannan antigen in sooty mangabey monkeys experimentally inoculated with mycobacterium leprae
ABSTRACT
IgG and IgM antibody levels to mycobacterial lipoarabinomannan (LAM) antigen were determined by ELISA in eight sooty mangabey monkeys (Cercocebus atys) prior to and at intervals after experimental inoculation with Mycobacterium leprae. High levels of anti-LAM IgG were present before inoculation and increased thereafter in the five mangabeys that developed lepromatous (LL) forms of leprosy; lower levels of anti-LAM IgG were observed in two mangabeys that developed indeterminate leprosy and tuberculoid/neuritic leprosy, respectively, and in a mangabey that was leprosy resistant. IgM anti-LAM levels were near zero before M. leprae inoculation in all eight animals, rose significantly in only three LLleprosy-susceptible animals after inoculation, and returned to near zero in all animals within 3 years.
Anti-LAM antibody levels appear to be potentially valuable as an indicator of leprosy susceptibility, and when measured longitudinally together with antibody levels to M. leprae-specific phenolic glycolipid-I antigen, as a means to detect preclinical M. leprae infections in high-risk individuals.
RÉSUMÉ
Chez huit singes mangabey (Cercocebus atys), on a déterminé au moyen d'une méthode ELISA les taux d'anticorps IgG et IgM au lipoarabinomannan mycobactérien (LAM), avant inoculation expérimentale par Mycobacterium leprae, et ensuite à intervalles répétés après cette inoculation. Des taux élevés d'IgG-LAM étaient décelables avant l'inoculation; ces taux ont ensuite augmenté chez les cinq singes ayant développé des formes lépromateuses (LL) de la lèpre. Des taux plus faibles d'IgG anti-LAM ont été relevées chez les deux singes mangabey qui avaient développé respectivement une lèpre indéterminée, et une lèpre tuberculoïde de type névritique, de même que chez un singe qui était résistant à la lèpre. Les taux d'IgM anti-LAM étaient proches de zéro avant inoculation par M. leprae, et ceci chez les huit animaux. Ces taux ont alors augmenté de manière significative après l'inoculation chez trois animaux susceptibles à la lèpre lépromatcusc; ils sont retournés à des niveaux proches de zéro chez tous les animaux dans les trois années qui ont suivi.
Les taux d'anticorps antiLAM semblent constituer un indicateur peutêtre intéressant de la susceptiblité à la lèpre. Lorsqu'on les mesure de manière longitudinale, en même tempsque les taux d'anticorps à l'antigène phénoglycolipidique-I spécifique pour M. leprae, ils pourraient permettre de déceler des infections précliniques à M. leprae chez des individus à haut risque.
RESUMEN
Usando un método inmunoenzimático (ELISA) se determinaron los niveles de anticuerpos IgG e IgM contra la lipoarabinomanana (LAM) micobacteriana en 8 monos mangabey pardos (Cercocebus atys) antes y a intervalos después de la inoculación experimental con Mycobacterium leprae. En 5 monos que desarrollaron lepra lepromatosa se encontraron niveles elevados de IgG anti-LAM antes de la inoculación con M. leprae y éstos aumentaron después de la infección; en 2 monos que desarrollaron lepra indeterminada a tuberculoide/neurítica y en un mono que fue resistente a la lepra, se observaron niveles más bajos de IgG anti-LAM. Los niveles de IgM anti-LAM fueron cercanos a cero en 8 monos antes de la inoculación con M. leprae, en 3 animales susceptibles (lepromatosos) los niveles de IgM aumentaron significativamente después de la inoculación y regresaron a valores cercanos a cero en todos los animales dentro de los siguientes 3 años.
Los anticuerpos anti-LAM parecen ser potencialmente valiosos como indicadores de susceptibilidad a la lepra, y cuando se miden paralelamente con los anticuerpos antiglicolipido fenólicoI del M. leprae, resultan adecuados para detectar infecciones preclinicas por M. leprae en individuos de alto riesgo.
There is considerable interest in the possibility that antibody assays for antigens of Mycobacterium leprae might provide a means of early detection of subclinical leprosy (4, 8, 9, 11, 16, 18). It is also important to determine if leprosy-susceptible individuals can be identified among groups in leprosyendemic areas.
The availability of purified forms of mycobacterial carbohydrate-containing antigens, such as lipoarabinomannan (LAM) and the M. leprae-specific (12,14,15) phenolic glycolipid-I (PGL-I) antigen, has made it possible to perfect serologic assays for the determination of antibodies to these cell-wall constituents (1, 6, 16). The serodominant epitope of the PGL-I antigen has been found to reside in the terminal trisaccharide region of the molecule; synthetic glycoconjugates bound to bovine serum albumin (BSA) arc available for use in ELISA for detection of anti-PGL-I antibodies (1, 2, 5, 6, 12, 14, 15).
Several studies utilizing these serologic methods have suggested that antibody titers to PGL-I can be used to identify the majority of multibacillary (MB) leprosy patients and lesser numbers of paucibacillary (PB) subjects (1, 2, 5, 6, 17, 20, 22). Some studies suggest that antibody titers to PGL-I determined by ELISA may be useful in identifying subclinical leprosy patients among leprosy household contacts (3, 18, 21), but this point is disputed (11).
In an ELISA study of IgG and IgM antibody titers to LAM and PGL-I, Levis, et al. found that 71% of PB and 85.5% of MB patients were positive for at least one of the following three antibodies: IgG anti-LAM, IgM anti-LAM, or IgM anti-PGL-I; one symptomatic contact with a neuropathy was positive for anti-LAM and anti-PGL-I IgM and for IgG antibodies to PGL-I (16). These results suggest that it might be important to monitor each of these antibody types in order to detect most M. leprae-infected individuals.
We recently observed that monitoring IgG and IgM antibody levels to PGL-I in eight sooty mangabey monkeys (Cercocebus atys) experimentally inoculated with M. leprae indicated that elevated anti-PGL-I IgG correlated with resistance to and elevated anti-PGL-I IgM with susceptibility to leprosy (13). In the present study, we monitored the IgG and IgM antibody levels to LAM in these same eight mangabeys to test the suggestion of Levis, et al. that IgG and IgM antibody titers to LAM and PGL-I can be used together for diagnosing leprosy, and to determine if LAM antibody levels are related to disease susceptibility (16).
MATERIALS AND METHODS
Animals and M. leprae inoculations. We have previously reported the details concerning the sources of animals, routes of inoculation, criteria for disease classification, and descriptions of leprosy progression in the four pairs of mangabeys studied here (13). In brief, mangabeys D171 and D172 were inoculated with the highest dose of M. leprae (4.8 × 1010 bacilli, 10% morphologic index); serial, tenfold dilutions were given in a paired fashion to D173 and D174, D175 and D176, and D177 and D178, respectively. Monkey sera were obtained at the times indicated and stored at -70ºC until assayed.
It is noteworthy that the animals were age-and sex-matched (males) and were born in captivity in a group compound at the Yerkes Regional Primate Research Center (Atlanta, Georgia, U.S.A.). After shipment to the Delta Regional Primate Research Center (Covington, Louisiana, U.S.A.) in 1982 at approximately 2 years of age, the animals were housed in individual cages.
Leprosy classifications. All classifications of leprosy are according to the Ridley and Jopling system (19).
ELISA. LAM was purified from M. tuberculosis and provided under NIAID contract #l-AI-52582 by Dr. Patrick J. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A. LAM was dissolved in carbonate buffer at 0.5 μg/ml. Flatbottom, 96-well plates (Immulon I; Dynatech Laboratories, Inc., Chantilly, Virginia, U.S.A.) were used, and a Dynatech MR-700 ELISA reader was used to determine the final optical density (OD) values. Monkey sera were assayed at the predetermined optimal dilutions of 1:200 for IgM and 1:100 for IgG.
Anti-LAM levels were determined after adding 50 μl of LAM or carbonate buffer alone to experimental or control wells, respectively, and incubation of the plates at 4ºC overnight. After emptying, 100 μl of 1% bovine serum albumin-phosphate buffered saline (BSAPBS), pH 7.4, was added to each well and the plates were incubated for 1 hr at 37ºC. The plates were then washed with PBS and incubated with 50 μl of monkey antiserum diluted in PBS10% heatin-activated (56ºC, 30 min) normal goat serum for 1 hr at 37ºC followed by washing four times in PBS. Fifty μl ofoptimally diluted (1:1000, IgG; 1:500, IgM) peroxidase-conjugated, goat anti-human IgG or IgM (anti-Fc piece, μ or γ-specific, affinity purified; Cooper Biomedical, Malvern, Pennsylvania, U.S.A.) was then added toeach well. The plateswere incubated for 1 hr at 37ºC followed by four washes in PBS. Thereafter, 50 μl of O-phenylenediamine (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) at 0.4 mg/ml in 0.2 M citratephosphate buffer, pH 5.0, containing 0.002% H2O2, was added to each well. The wells were acidified with 50 μl of 2.5 N H2SO4, and the OD at 490 nm was determined. The reported OD values represent the OD ofwells containing LAM antigen minus that of control wells lacking LAM antigen but containing all other components.
Statistics. AntiLAM OD values for groups of animals with lepromatous (LL) leprosy, those with paucibacillary disease, and one of the M. leprae-inoculated mangabeys that remained healthy were subjected to an analysis of variance. The results showed that values for paucibacillary leprosy could be grouped together with the leprosyresistant M. leprae-inoculated mangabey, and that the data from the five animals with LL leprosy could also be grouped. A comparison of mean values for the two groups by the analysis of variance showed that OD values for the LL group differed significantly from that of the other group (p < 0.0001). Further comparison of the two groups by the Student's t test confirmed that level of significance.
RESULTS
Figure 1A shows the time course of the appearance ofserum IgG antibody to LAM in each of the eight mangabeys beginning prior to M. leprae inoculation. Five of the eight animals (D171, D172, D174, D176, and D177) ultimately developed lepromatous (LL) leprosy. Preinoculation levels of IgG antibody to LAM were highest in these 5, compared to the other 3 animals, and each ofthe 5 developed antiLAM IgG levels in excess of 1.2 OD units after M. leprae inoculation.
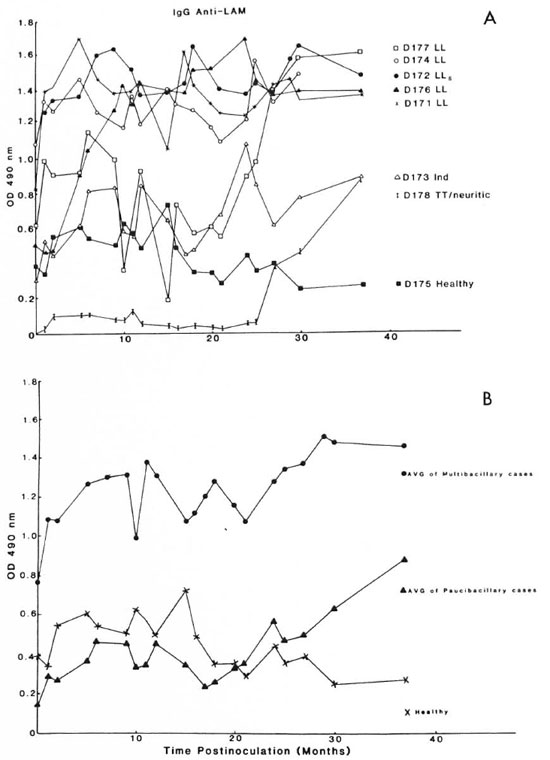
Fig. 1. Serum IgG antibody levels to lipoarabinomannan (LAM) antigen in mangabey monkeys before and at intervals after M. leprae inoculation. A = The indicated mangabeys (D171, D172, D174, D176, and D177) developed lepromatous (LL) or subpolar lepromatous (LLs) leprosy; D173 developed indeterminate leprosy; D178 developed TT/neuritic leprosy; D175 remained healthy. B = Average values for the group of animals that developed LL (multibacillary) forms ( ) or indeterminate and TT/neuritic (paucibacillary) forms (
) or indeterminate and TT/neuritic (paucibacillary) forms ( ) of leprosy and the values for the animal that failed to develop leprosy (×).
) of leprosy and the values for the animal that failed to develop leprosy (×).
Two (D173 and D178) of the remaining three mangabeys developed paucibacillary forms of leprosy (indeterminate and tuberculoid/neuritic) and the other animal (D175) has remained healthy to date. These latter three mangabeys each had preinoculation levels of anti-LAM IgG no greater than 0.4 OD unit, and the levels remained below 1.0 OD unit throughout the course of observation after M. leprae inoculation except for a single transient fluctuation above that value (Fig. 1A). Mangabeys D171, D172, and Dl76 developed clinical signs of LL leprosy by 4-6 months, D174 by 10 months, and D177 by 26 months postinoculation; D173 showed clinical signs of paucibacillary leprosy by 12 months and D178 by 35 months postinoculation (13). In each animal the preinoculation level of anti-LAM IgG had begun to increase prior to the clinical appearance of leprosy (Fig. 1A).
Figure 1B shows the average ELISA level for anti-LAM IgG in the group of five mangabeys with multibacillary (LL) leprosy, the two animals with paucibacillary leprosy (indeterminate and TT/neuritic), and the healthy mangabey. The average values clearly show that mangabeys susceptible to multibacillary (LL) forms of leprosy have elevated levels of anti-LAM IgG prior to M. leprae inoculation, and the levels rapidly increase after inoculation in conjunction with progression to LL leprosy (13). Mangabeys that will ultimately develop paucibacillary forms of leprosy have much lower baseline anti-LAM IgG levels that gradually begin to increase after inoculation and prior to the appearance of clinical signs of leprosy (13) (Fig. 1B). The leprosy-resistant mangabey (D175) had a preinoculation anti-LAM IgG level of 0.4 OD unit which failed to undergo a significant sustained change (Fig. 1B).
Figure 2 shows the IgM anti-LAM ELISA data. Preinoculation levels were below 0.1 OD unit in each of the 8 mangabeys, and the levels rose transiently above 0.2 OD units after M. leprae inoculation in only 3 out of 5 of the animals that developed LL forms of leprosy (D171, D174, and D176). IgM anti-LAM levels ultimately fell to below 0.1 OD unit in all eight mangabeys within 3 years after M. leprae inoculation. There were no significant detectable anti-LAM IgM responses in the leprosy-resistant, healthy animal (D175), the two mangabeys with paucibacillary leprosy (D173 and D178), and two of those with LL leprosy (D172 and D177) (Fig. 2).
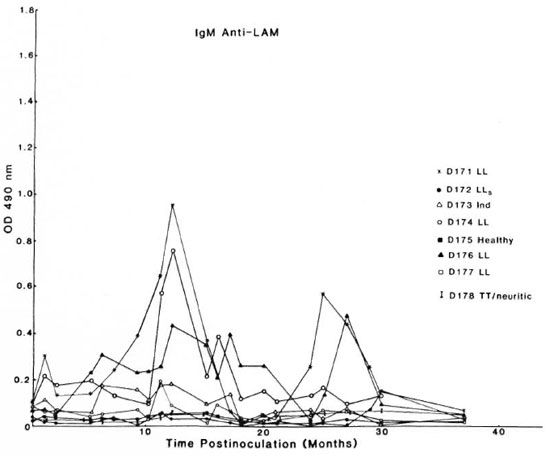
Fig. 2. Serum IgM antibody levels to lipoprabinomannan (LAM) antigen in mangabey monkeys before and at intervals after M. leprae inoculation (see Fig. 1A legend for details).
DISCUSSION
The data are consistent with the observations of Levis, et al. that anti-LAM IgG antibody levels are useful for detecting individuals with multibacillary and paucibacillary leprosy (16). Elevated levels of anti-LAM IgG were observed in each of the five mangabeys that developed LL forms of leprosy months before the appearance of clinical signs of leprosy (13). Significantly, the five LL leprosy-susceptible mangabeys had the highest levels of anti-LAM IgG of the eight mangabeys studied, greater than 0.5 OD unit, prior to M. leprae inoculation. After inoculation, the IgG anti-LAM level increased to and remained greater than 1.2 OD unit in each of the five animals with LL leprosy.
The two mangabeys that developed paucibacillary forms of leprosy (D173, indeterminate; D178, tuberculoid/neuritic) had anti-LAM levels of 0.32 and near 0 OD units, respectively, prior to M. leprae inoculation; the levels increased gradually, beginning several months prior to the appearance of clinical symptoms and reaching maximal levels above 0.8 OD unit after the appearance of disease (13). Mangabey D175, which has failed to develop signs of leprosy (13) had a preinoculation anti-LAM IgG level of 0.4 OD unit which has fluctuated but remained near the preinoculation level 3 years after M. leprae inoculation.
These results, together with our previous observations on IgG and IgM anti-PGL-I antibody levels (13), confirm that of the four antibody types studied, IgG and IgM anti-PGL-I and IgG and IgM anti-LAM, at least one will be detectably elevated in monkeys after infection with M. leprae and prior to the appearance of signs of the disease (16). This is markedly so in the case of animals that will develop LL forms of leprosy. It should be noted, however, that our previous data showed that elevated IgG anti-PGL-I levels, especially in the absence of significant IgM anti-PGL-I antibody levels, correlate with resistance to the development of leprosy although a subclinical infection may be present (13).
Anti-LAM IgM levels increased significantly but transiently in only 3 of the 5 LL-susceptible mangabeys. The remaining animals studied snowed no significant IgM anti-LAM levels. These observations suggest that anti-LAM IgM antibodies appear to play little role in the pathogenesis of leprosy.
Our observations indicate that anti-LAM IgG levels are much more sensitive in the detection of preclinical leprosy and/or susceptibility to the disease than the determination of anti-PGL-I IgG or IgM levels. Anti-PGL-I IgG or IgM levels arc much more specific for leprosy detection, however, inasmuch as all eight mangabeys studied had significant anti-PGL-I IgG or IgM levels at some point after M. leprae inoculation but no detectable anti-PGL-I IgG or IgM levels prior to M. leprae inoculation (13). This pattern of specificity is not surprising since LAM antigen may be common to most if not all mycobacterial species, whereas PGL-I appears to be a specific M. leprae antigen (12, 14, 15). It is significant, however, that the mangabeys with anti-LAM IgG levels greater than 0.5 OD prior to inoculation were all susceptible to LL forms of leprosy; whereas the remaining three animals each had an anti-LAM IgG level below 0.5 OD and each of these failed to develop multibacillary leprosy. This result suggests the possibility that prior exposure to LAM-containing environmental mycobacteria may play a role in increasing the susceptibility to leprosy in at least some individuals. Each of the animals studied, however, was age-and sex-matched and had similar histories. They were born and grouphoused in the same compound, followed by later housing in individual cages in the same room. They each had the same sources of food and water. It seems likely, therefore, that each animal might have been exposed to the same sources of environmental mycobacteria. Further study will be necessary to elucidate the detailed nature of the relationship between anti-LAM IgG levels and susceptibility to leprosy.
Pronounced differences in leprosy susceptibility were observed between pairs of mangabeys that were inoculated by similar routes with identical numbers of M. leprae (13). Thus, whatever the mechanism(s) may be, the results arc consistent with the possible involvement of underlying genetic influences in addition to possible environmental factors (11).
One practical consideration resulting from our observations is that in leprosy-endemic areas, asymptomatic individuals with elevated anti-LAM IgG titers alone or especially in combination with anti-LAM IgM or anti-PGL-I IgG or IgM might be advised to undergo prophylactic multidrug or vaccine therapy (7). Asymptomatic individuals who possess such antibody profiles and who live in very close contact with LL leprosy patients might be especially advised to consider prophylactic therapy.
It should be noted that in the present study we had the luxury of being able to study the monkeys prior to M. leprae infection, to know precisely when infection occurred, and to obtain serum samples and observe the animals longitudinally as leprosy developed and progressed. The mangabeys were born and reared in the U.S.A. and, in all probability, had never been exposed to M. leprae prior to experimental inoculation. This combination of circumstances is not-readily possible in human populations. One cannot know whether an individual in a leprosyendemic area has been subclinically infected with M. leprae or another mycobacterium. This presents a problem in defining what a "normal" or unexposed baseline level of anti-PGL-I or anti-LAM antibody titer might be among any given human population. For this reason, it is necessary to perform longitudinal serological ELISA for IgG and IgM antibody levels to PGL-I and to LAM in order to monitor and screen human groups who are at risk for leprosy infection.
Human populations in leprosy-endemic areas should be carefully studied to determine whether our observations apply to humans. Previous studies in human leprosy patients suggest that similar relationships may exist (16), A minimal ELISA screen for IgG antibody to LAM might be recommended; follow up anti-PGL-I IgM determinations would be suggested for those who test positive for anti-LAM. Our observations in mangabey monkeys suggest that individuals with high and increasing levels of IgG anti-LAM and IgM anti-PGL-I and low or absent IgG anti-PGL-I arc highly susceptible and at risk to develop LL leprosy(13).
Acknowledgments. We are indebted to Mrs. Cyndi Trygg for technical assistance and to Mrs. Mary Ann Bennett for typing this manuscript. Graphics were provided by Mr. Murphey Dowouis. Financial support was provided by grants from the National Institutes of Health #RR-00164, the National Institute for Allergy and Infectious Diseases #2R22AI19302 and from the Leonard Wood Memorial Leprosy Foundation. The sooty mangabeys were provided by Dr. Harold McClure of the Yerkes Regional Primate Research Center, Atlanta, Georgia, U.S.A.
REFERENCES
1. BRETT, S. J., DRAPER, P., PAYNE, S. N. and REES, R. J. W. Serological activity of a characteristic phenolicglycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271279.
2. BRETT, S. J., PAYNE, S. N., GIGG, J., BURGESS, P. and GIGG, R. Use of synthetic glycoconjugates containing Mycobacterium leprae-specific and immunodominant epitope of phenolic-glycolipid-I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476483.
3. BUCHANAN, T. M., DISSANAYAKE, S., YOUNG, D. B., MILLER, R. A., ACEDO, J. R., HARNISCH, J. P., KHANOLKAR, S. R. and ESTRADAPARRA, S. Evaluation ofthe significance ofantibodies to phenolic glycolipid of Mycobacterium leprae in leprosy patients and their contacts. Abstract in Int. J. Lepr. 51(1983)658659.
4. CHANTEAU, S., CARTEL, J.-L., GUIDI, C , PLICHART, R. and BACH, M.-A. Seroepidemiological study on 724 household contacts of leprosy pa tients in French Polynesia using disaccharide-octyl-BSA as antigen. Int. J. Lepr. 55(1987)626-632.
5. CHO, S.N., FUJIWARA, T., HUNTER, S. W., REA, T. H., GELBER, R. H. and BRENNAN, P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-β-glucopyranosyl epitope for the serodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
6. CHO, S.N., YANAGIHARA, D.L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
7. CONVIT, J., ULRICH, M., ARANZAZU, N., CASTELLANOS, P. L., PINARDI, M. E. and REYES, O. The development of a vaccination model using two microorganisms and its application in leprosy and leishmaniasis. Lepr. Rev. 57 Suppl.2(1986)263-273.
8. FINE, P. E. M. Problems in the collection and analysis of data in leprosy studies. Lepr. Rev. 52 Suppl.(1981)197-206.
9. FINE, P. E. M. Leprosythe epidemiology of a slow bacterium. Epidemiol. Rev. 4(1982)161-188.
10. FINE, P. E. M. Implications of genetics for the epidemiologyandcontrol ofleprosy. Philos. Trans. R. Soc. Lond. [Biol.] 321(1988)365-376.
11. FINE, P. E. M., PONNINGHAUS, J. M., BURGESS, P., CLARKSON, J. A. and DRAPER, C. C. Seroepidemiological studies of leprosy in northern Malawi based on an enzymelinked immunosorbent assay using a synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
12. GAYLORD, H. and BRENNAN, P. J. Leprosy and the leprosy bacillus: recent developments in characterization of antigens and immunology of the disease. Annu. Rev. Microbiol. 41(1987)645-657(139 ref.).
13. GORMUS, B. J., OHASHI, D. K., OHKAWA, S., WALSH, G. P., MEYERS, W. M., BRENNAN, P. J. and TRYGG, C. Serologic responses to Mycobacterium leprae-specific phenolic glycolipid-I antigen in sooty mangabey monkeys with experimental leprosy. Int. J. Lepr. 56(1988)537-545.
14. HUNTER, S. W., FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)10572-10578.
15. HUNTER, S. W., GAYLORD, H. and BRENNAN, P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tuberculosis bacilli. J. Biol. Chem. 261(1986)12345-12351.
16. LEVIS, W. R., MEEKER, H. C, SCHULLER-LEVIS, G., SERSEN, E., BRENNAN, P. J. and FRIED, P. J. Mycobacterial carbohydrate antigens for serological testing of patients with leprosy. J. Infect. Dis. 156(1987)763-769.
17. LEVIS, W. R., MEEKER, H. C, SCHULLER-LEVIS, G., SERSEN, E. and SCHWERER, B. IgM and IgG antibodies to phenolic glycolipid I from Mycobacterium leprae in leprosy: insight into patient monitoring, erythema nodosum leprosum, and bacillary persistence. J. Invest. Dermatol. 86(1985)529-534.
18. MENZEL, S., HARBOE, M., BERGSVIK, H. and BRENNAN, P. J. Antibodies to a synthetic analog of phenolic glycolipid-I of Mycobacterium leprae in healthy household contacts of patients with leprosy. Int. J. Lepr. 55(1987)617-625.
19. RIDLEY, R. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
20. SCHWERER, B., MEEKER, H. C, SERSEN, G. and LEVIS, W. R. IgM antibodies against phenolic glycolipid-I from Mycobacterium leprae in leprosy sera: relationship to bacterial index and erythema nodosum leprosum. Acta Leprol. 2(1984)395-402.
21 . ULRICH, M., CONVIT, J. and CENTEN, O. M. Antibody to soluble antigen of M. leprae and phenolic glycolipid-I in patients with leprosy and contacts. Abstract in Int. J. Lepr. 52 Suppl.(1984)692.
22. YOUNG, D. B., DISSANAYAKE, S., MILLER, R. A., KHANOLKAR, S. R. and BUCHANAN, T. M. Humans respond predominantly with IgM immunoglobulin to the species-specific glycolipid of Mycobacterium leprae. J. Infect. Dis. 149(1984)870-873.
1. Ph.D., Research Scientist, Tulane University, Three Rivers Road, Covington, Louisiana 70433, U.S.A.
2. M.D., Delta Regional Primate Research Center, Tulane University, Three Rivers Road, Covington, Louisiana 70433, U.S.A.
3. M.D., Ph.D., Chief, Mycobacteriology, Armed Forces Institute of Pathology, Washington, D.C 20306, U.S.A.
4. Ph.D., Leonard Wood Memorial Laboratory for Leprosy Research, P.O. Box 727, Cebu City, The Philippines.
5. M.D., Bayley Seton Hospital, Staten Island, New York, New York 10304, U.S.A.
6. Ph.D., Bayley Seton Hospital, Staten Island, New York, New York 10304, U.S.A.
Received for publication on 30 May 1989.
Accepted for publication in revised form on 16 August 1989.