- Volume 58 , Number 1
- Page: 121–3
Blister calendar packs for dapsone monotherapy
To the Editor:
In 1982, the World Health Organization (WHO) published their recommendations for the treatment of all cases of leprosy with multiple drug therapy (MDT) in regimens of relatively short duration (7). Since that date, MDT has been widely applied in the majority of leprosy-endemic countries, and by the time of the XIII International Leprosy Congress in The Hague (4), WHO was able to report that by mid-1988, over 2 million of the approximately 5 million registered cases had been put on MDT, and that of those, over a quarter had completed treatment and were no longer considered to have active leprosy. On the basis of numerous publications and reports, it is now clear that the regimens advised are operationally feasible, acceptable to patients and health staff, clinically and bacteriologically effective, and not attended by an undue incidence of toxic effects or adverse immunological reactions. Most importantly, relapse rates for either paucibacillary or multibacillary cases have been remarkably low in the follow-up periods so far. MDT, properly applied, is capable of reducing prevalence rates by about 75% within 5-10 years, while at the same time reducing child and disability rates, and-in the somewhat longer term-incidence (5).
All of this is tremendously encouraging, and it is now clear that most people working in leprosy control are concentrating on the implementation of MDT as the most decisive tool available for this purpose. However, in this letter I would like to look at what one might call "the other side of the coin" and to ask if more serious attention should perhaps be given to the very large numbers of patients who are receiving a form of treatment (dapsone monotherapy) which was condemned well over a decade ago as being unsatisfactory and hazardous, mainly because of the risks of resistance. From the world total of registered (known) patients of 5.1 million (6) about 32% are currently on MDT. This obviously leaves about 68% who are not on MDT, and although precise information is (to my knowledge) not available, the likelihood is that the majority are taking dapsone monotherapy. An additional concern is that "dapsone monotherapy programs," with some notable exceptions, tend to be characterized by poor organization, weak supervision and defective operational support. The latter, at least in my experience, frequently includes defects in the ordering and dispensing of dapsone tablets, and in their presentation to patients in a manner which is likely to achieve regular daily intake, in the correct dosage, over adequate periods of time.
In 1983, in the Correspondence section of this journal, a letter was published advocating the use of "bubble" or "calendar" packs for antileprosy drugs (8). Diagrams were presented for the use of multiple drugs in the regimens recommended by WHO (9), but we also included three designs for the administration of dapsone alone, using either figures, days of the week, or phases of the moon as a guide to daily intake for patients of differing cultures and educational levels. One of these is reproduced here (The Figure).
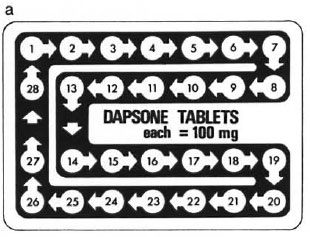
The Figure. Diagram (a), reproduced from reference (5), of a blister-calendar pack for 1 month's supply(28 days) of tablets of dapsone, each 100 mg, for unsupervised daily self-administration by the patient on dapsone monotherapy. Dimension = 10 × 7 cm.
Despite the absence of published data so far which indicate that blister calendar packs (BCPs) have definite advantages over the issue of drugs "loose" or that they are costbeneficial, they are nevertheless in widespread use. In 1985, with support from the Sasakawa Memorial Health Foundation and WHO, BCPs were introduced in The Philippines for the implementation of MDT, using WHO regimens (9). The use of packs locally produced by The Leprosy Mission in southern Africa, and the production and distribution of packs by Ciba-Geigy in Basle and Pharmanova in Copenhagen, has been described in a recent editorial in this journal (2). It has recently been reported (1) that 49,500 leprosy patients in India have already received MDT in this form, and it is expected that about 1.7 million packs will be produced over a 4-5 year period. The British Leprosy Relief Association (LEPRA) is also using them in MDT projects in India, and packs from Ciba-Geigy have been distributed to many control programs in South America, Africa, South-East Asia, and the Far East.
There is certainly much more behind the success of MDT programs than the drugs or the way they arc presented to patients, and BCPs cannot be expected to solve all of the problems, including those which currently impede the wider implementation of MDT, notably in Africa. In the case of dapsone monotherapy programs, however, my impression is that the present situation (involving large numbers of patients) is unnecessarily bad and that it could be improved by a) the presentation of dapsone in BCPs, using a pack such as that shown in The Figure, and b) the addition of locally produced, written, and illustrated material, such as that recently described (3), giving clear instructions and encouragement to the patient in appropriate terms. Dapsone tablets arc universally available in a standard size, and the construction of pack of 28 tablets, each of 100 mg, could be carried out either locally or by an international drug company with minimal outlay in money and machinery. We seem to be faced with the fact that many patients with leprosy have to take dapsone monotherapy, perhaps for some years to come. Would it not be wise to do all that is possible to ensure that they take it regularly, in the correct dose, and for adequate periods of time?
- A. Colin McDougall, M.D., F.R.C.P.
87 Lower Radley Near Abingdon
Oxfordshire OX 14 3BA, U.K.
REFERENCES
1. A new approach to multidrug therapy for leprosy. (Editorial) Afr. Health 11(1989)5-6.
2. GEORGIEV, G. D. and MCDOUGALL, A. C. Blister calendar packs-potential for improvement in the supply and utilization of multiple drug therapy in leprosy control programs. (Editorial) Int. J. Lepr. 56(1988)603-610.
3. MCDOUGALL, A. C. and GEORGIEV, G. D. Educational material for the patient with leprosy. Lepr. Rev. 60(1989)221-228.
4. Transactions of the Thirteenth International Leprosy Congress. Int. J. Lepr. 57(1989)229-444.
5. UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES (TDR). Tropical diseases; progress in international research, 1987-1988 . Geneva: World Health Organization, 1989.
6. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768 .
7. WHO STUDY GROUP. Chemotherapy ofleprosy for control programmes. Geneva: World Health Organization, 1982 . Tech. Rep. Ser. 675 .
8. WINSLEY, B. E., MCDOUGALL, A. C. and BROWN, K. E. Chemotherapy of leprosy: "bubble" or "calendar" packs for the administration of rifampin, dapsone, clofazimine or prothionamide/ethionamide. (Letter) Int. J. Lepr. 51(1983)592-594.
9. YUASA, Y. Operational considerations in multiple drug therapy (MDT) implementation. In: Regional Workshop on Multiple Drug Therapy (MDT), Cebu, The Philippines, Sept./Oct. 1987.