- Volume 58 , Number 1
- Page: 126–8
Detection of interleukin-2 Receptor (IL-2r) by indirect immunofluorescence with anti-Tac monoclonal antibody on the surface of T lymphocytes from patients with lepromatous leprosy
To the Editor:
We have recently published that lymphocytes from patients with lepromatous leprosy (LL) stimulated with concanavalin A (ConA) are deficient in the synthesis of interleukin-2 (IL-2); however, the cells possess receptors for IL-2 (IL-2r) in such a manner that when activated lymphocytes are cultured in vitro and exogenous IL-2 is added, they recover their capacity to proliferate as demonstrated by the incorporation of tritiated thymidine (3). A demonstration of the physical presence of IL-2r on the surface of T lymphocytes activated with phytohemaglutinin (PHA) by means of indirect immunofluorescence (IIF) using an anti-Tac monoclonal antibody (4, 8, 9, 11) is reported here. (The anti-Tac monoclonal antibody was kindly provided by Dr. T. A. Waldmann, Metabolism Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, U.S.A., and we thank him for his courtesy.)
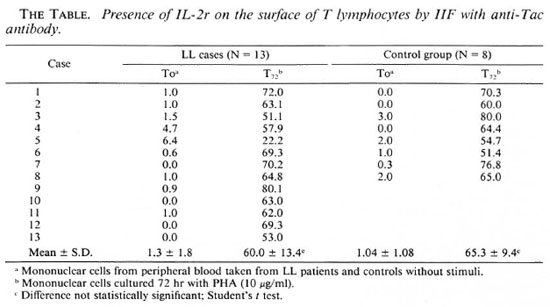
Five women and eight men between the ages of 20 and 60 years old were studied at the Instituto Dermatologico de Guadalajara, Jalisco, Mexico. All of them had nodular or diffuse LL, a positive bacilloscopy, and had been receiving irregular treatment with 100 mg of dapsone daily for from 2 to 15 years. The control group consisted of eight healthy persons, of similar sex and age, not related to the patients.
The mononuclear cells from heparinized venous blood were obtained by means of Ficoll-Hypaque and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum (3). The IL-2r was studied and detected by IIF (1) on the membrane of the lymphocytes using anti-Tac monoclonal antibody; for this purpose two groups of cells were studied. The first group (To) corresponds to lymphocytes without any stimulation; the second group (T72) consisted of lymphocytes previously activated with PHA for 72 hr. Cells (1 × 106) obtained from experiments To and T72 were incubated for 30 min at 4ºC with anti-Tac monoclonal antibody diluted 1:500. The cells were then washed and again incubated for 30 min at 4ºC with anti-mouse IgG antibody labeled with fluorescein isothiocyanate (FITC) (Sigma Chemical Co., St. Louis, Missouri, U.S.A.), and were analyzed by fluorescence microscopy. Data were obtained by counting 300 cells and the percent of Tac+ cells was calculated for each of the studied groups.
The data obtained by IIF show that both the cells from the group of LL patients and those of the control group not activated with PHA (To) had practically no Tac+ cells (The Table). However, in both groups after activation with PHA for 72 hr (T72) the percentages were 60.8 ± 13.4 for cells from LL subjects and 65.3 ± 9.4 for the control group. These results were not different statistically when an independent Student's t test was performed.
The Figure. Tac + cells revealed by indirect immunofluorescence. Anti-Tac monoclonal antibodies are bound to IL-2r and demonstrated by fluorescent anti-mouse IgG antibodies.
This study demonstrates the physical presence of the IL-2r by the specific union of anti-Tac monoclonal antibodies to the 55-kDa chain of the high-affinity IL-2r (6-9), and confirms that T cells of LL patients truly possess IL-2r but are incapable of proliferating adequately due to the deficiency of IL-2 biosynthesis (2-4). It should be mentioned that Mohagheghpour, et al. found similar results using ConA (5), but the percentages of T cells expressing IL-2r (Tac +) were lower than those found by us; this difference is probably due to the fact that PHA is a better inducer of IL-2r (11).
- Mary Fafutis-Morris, M.Sc.
Silvia Mejia-Arreguin, M.T.
Amado Gonzalez-Mendoza, M.D.
Unidad de Investigacion Biomedica de Occidente
Instituto Mexicano del Seguro Social
Division de Patologia Experimental
Sierra Mojado 800
Guadalajara, Jalisco, Mexico
- Roberto Morales-Ortiz, M.D.
Instituto Dermatologico de Guadalajara SSA
Guadalajara, Jalisco, Mexico
-Alfonso Islas-Rodriguez, M.Sc.
Instituto de Patologia Infecciosa y Experimental
Universidad de Guadalajara
Guadalajara, Jalisco, Mexico
REFERENCES
1. COONS, A. H. Histochemistry with labeled antibody. Int. Rev. Cytol. 5(1956)1-4.
2. HAREGEWOIN, A., MUSTAFA, A. S., HELLE, I., WATERS, M. F., LEIKER, D. L. and GODAL, T. Reversal by interleukin-2 of the T-cell unresponsiveness of lepromatous leprosy to Mycobacterium leprae. Immunol. Rev. 80(1984)76-86.
3. ISLAS, R. A., MORALES, O. R., FAFUTIS, M. M., GONZALEZ, M. A. and ORTIZ, O. L. Deficiency in the biosynthesis of interleukin-2 (IL-2) and functional presence of the IL-2 receptor in lepromatous leprosy. Int. J. Lepr. 55(1987)566-569.
4. LEONARD, W. J., DEPPER, J. M., UCHIYAMA, T., SMITH. A. and WALDMANN, T. A. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characteriza tion of the receptor. Nature 300(1982)267-269.
5. MOHAGHEGHPOUR, N., GELHER, R. H., LARRICK, J. W., SASAKI, D. T., BRENNAN, P. J. and ENGLEMAN, E. G . The effective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with the defective expression of in terleukin-2 receptors and is not reconstituted by interleukin-2. J. Immunol. 135(1985)1433-1449.
6. NOGUEIRA, N., KAPLAN, G. , LEVY, E., SARNO, E. N., KUSHNER, P., GRANELLI-PIPERNO, A., VIEIRA, L., GOULD, V. C, LEVIS, W., STEINMAN, R., YIP. Y. K. and COHN, Z. A. Defective γ interferon production in leprosy; reversal with antigen and interleukin 2. J. Exp. Med. 158(1983)2165-2170.
7. RIDLEY, D. S. and JOPLING, W . H . Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
8. ROBB, R. J., GREENE, N. C. and Rusj, C. M. Low and high affinity cellular receptors for interleu kin-2; implications for the level of Tac antigen. J. Exp. Med. 160(1983)1126-1146.
9. ROBB, R. J., MUNCK, A. and SMITH. K. A. T cell growth factor receptors; quantification, specificity, and biological relevance. J. Exp. Med. 154(1982)1455-1474.
10. SMITH, K. A. The 2-chain structure of high-affinity IL-2 receptors. Immunol. Today 8(1987)11-14.
11. UCHIYAMA, T., BRODER, S. and WALDMANN, T. A. I. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J. Immunol. 126(1981)1393-1397.