- Volume 58 , Number 1
- Page: 129–30
An attempt to demonstrate antinerve antibodies in leprosy sera using rabbit nerve as an antigen
To the Editor:
Leprosy is a disease in which peripheral nerves arc the main targets of parasitization by Mycobacterium leprae. As a result of this infection nerve tissues are damaged. Due to this nerve tissue damage some antigens may be sequestered and may be released in the body, which eventually may give rise to antinerve antibodies in the body fluids. Keeping this possibility in mind, attempts have been made by various laboratories to demonstrate antinerve antibodies in leprosy sera. Mshana, et al. (5) used bovine basic myelin protein in their assay, and they could not detect antinerve antibodies in leprosy sera. On the other hand, using human nerve antigen, Eustis-Turf, et al. (1) and Itty, et al. (2) could demonstrate antinerve antibodies in 38% and 100% of leprosy sera, respectively. Since human nerve antigen is not easily obtainable, in the present study we have used rabbit nerve antigen for demonstrating antinerve antibodies in leprosy sera. The rabbit nerve antigen was used in the present assay because a majority of the antigenic components of rabbit and human nerves are known to have antigenic similarities (3).
The nerve antigen used in the present study was prepared from sciatic nerves collected from rabbits. The connective tissue around the nerves was removed, and the nerves were washed thoroughly in 0.15 M phosphate buffered saline (PBS), pH 7.2. The nerve was then cut into small pieces which were ground in a glass homogenizer in PBS. The homogenate thus obtained was centrifuged at 2000 × g × 10 min. The supernatant was assayed for protein content by the method of Lowry, et al. (4), and stored at - 20ºC after aliquoting in small volumes.
The sera were collected from 76 clinically classified (6) leprosy patients attending the outpatient department of the Central JALMA Institute for Leprosy, Agra, India. The control group of sera was from 18 normal individuals working at the laboratories of the same institute. The groupwise distribution of the sera is shown in The Figure.
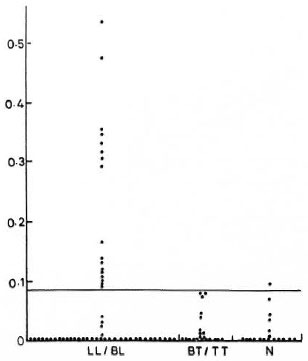
The Figure. Results (OD values) of ELISA for detection of antinerve antibodies in leprosy patients and healthy controls. Solid line indicates cut-off point, i.e., mean of OD values, in normal controls + 3 S.D. LL/BL = lepromatous leprosy/borderline lepromatous leprosy; TT/BT = tuberculoid leprosy/borderline tuberculoid leprosy; N = normal healthy controls.
Antinerve antibodies were quantitated by using an enzyme-linked immunosorbent assay (ELISA). Briefly, the wells of flat-bottom flexible microtiter plates (Dynatech Laboratories, Inc., Springfield, Virginia, U.S.A.) were coated with 50 μl (0.5 μg) of nerve antigen by overnight incubation at 4ºC. The wells were then washed three times with PBS containing 0.1 % Tween 20 (PBST). Thereafter, uncoated sites of the wells were blocked by adding 100 μl of 3% bovine serum albumin (BSA) in PBS. The plates were incubated at 37ºC for 1 hr and then washed again as described above. After this, 50 μl of the test serum (diluted 1:400 in 1% BSA-PBS to minimize the nonspecific background) was added to each well. The incubation was carried out at 37ºC for 45 min. The wells were then washed four times with PBST and 50 μl of 1:4000 diluted peroxidase-labeled antihuman IgG antibody (rabbit immunoglobulins; Dakopatts, Denmark) was added to each well. The plates were incubated at 37ºC for 30 min and the wells were washed four times in PBST. For development of color, 50 μl of the substrate (0.4 mg/ml of ortho-phenylene-diamine) was added to the wells, and incubation was carried out for 30 min at 37ºC. Finally, the enzymatic reaction was stopped by adding 50 μl of 2.5 N H2SO4 to the wells and the optical density (OD) values of the color developed were measured at 492 nm by a Multiskan ELISA Reader (Flow Laboratories, U.K.). The sera showing OD values above three standard deviations from the normal control group were taken as positive.
It was noted that 17 out of 54 (32%) lepromatous type patients were positive. None of the 22 tuberculoid type patients was found to be positive, and only 1 out of 18 normals was positive, according to our criteria.
It appears from our study using rabbit nerve antigen that only a minority of leprosy patients (22%) exhibit circulating antinerve antibodies. However, there was a significant difference (p < 0.05) between percent positivity in leprosy patients and normal controls. It will be interesting to search for antinerve antibodies in leprosy patients using nerve antigen from other laboratory animals, including primates.
- Om Parkash, M.Sc.
Assistant Research Officer
(Immunology)
- Sreevatsa, Ph.D.
Research Officer
(Experimental Leprosy)
- Utpal Sengupta, Ph.D.
Deputy Director
(Immunology)
Central JALMA Institute for Leprosy
Taj Ganj
Agra 282001, India
REFERENCES
1. EUSTIS-TURF, E. P., BENJAMINS, J. A. and LEFFORD, M.J. Characterization of the anti-neural antibodies in the sera of leprosy patients. J. Neuroimmunol. 10(1986)313-330.
2. ITTY, B. M., MUKHERJEE, R. and TALWAR, G. P. An enzyme immunoassay (EIA) based on antibodies against human nerve antigen for diagnosis of all categories of leprosy patients. Abstract in Int. J. Lepr. 57 Suppl.(1989)308 .
3. LATOV, N., GROSS, R. B., KASTELMAN, J., FLANAGAN, T., LAMME, S., ALKAITIS, D. A., OLARTE, H. R., SHERMAN, W. H., CHESS, L. and PENN, A. C. Complement-fixing antipheripheral nerve myelin antibodies in patients with inflammatory polyneuritis and with polyneuropathy and paraproteinemia. Neurology (N.Y.) 31(1981)1530-1534 .
4. LOWRY, O. H., ROSEBROUGH, N. J., FARR, A. L. and RANDALL, R. J. Protein measurement with Folinphenol reagent. J. Biol. Chem. 193(1951)265-275 .
5. MSHANA, R. N., HARBOE, M., STONER, G. L., HUGHES, R. A. C, KADLUBOWSKI, M and BELEHU, A. Immune response to bovine neural antigens in leprosy patients. I. Absence of antibodies to an isolated myelin protein. Int. J. Lepr. 51(1983)33-40.
6. RIDLEY, D. S. and JOPLING, W. H . Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1988)255-277.