- Volume 57 , Number 3
- Page: 599–606
Rate and time distribution of relapses in multibacillary leprosy
ABSTRACT
Of the 47,068 patients registered in the Polambakkam Leprosy Center between 1955 and 1982, we selected 1886 cases having shown bacteriological positivity at any time during this period, whatever their classifications at registration, and subsequently found bacteriologically negative. After an average follow-up period of 10 years, 243 relapses were observed, giving a crude relapse rate of 12.8 per person-years of observation and a cumulative probability of relapse of 18.9%. Relapse rates were found to be dependent on regularity during smear-positive and -negative periods; a regularity greater than 75% in the smear-positive period proved to be particularly important. The results show no evidence that relapses occurring after 3 years of negativity could be reinfections, and that the relapse rate was still affected by regularity 7 years after negativation. The median delay of relapses was found to be 4.4 years and was not affected by the regularity of treatment.RÉSUMÉ
Parmi les 47.068 patients enregistrés au Centre de Lèpre de Polambakkam entre 1955 et 1982, nous avons inclus dans l'étude 1886 cas qui furent positifs bacteriologiquement à n'importe quel moment durant cette période, quelque soit leur classification à l'enregistrement, et qui se sont négatives par la suite. Après un suivi d'une durée moyenne de 10 ans, 243 rechutes furent observées, donnant un taux de rechute global de 12,8 pour mille personne-années d'observation et une probabilité cumulative de rechute de 18,9%. L'analyse a démontré que le taux de rechute était influencé par la régularité au traitement pendant les périodes de positivité et de négativité; une régularité supérieure à 75% pendant la phase de positivité est particulièrement importante. Les résultats ne démontrent pas que les rechutes découvertes après 3 ans de négativité pourraient être des réinfections, et indiquent que le taux de rechute est encore influencé par la régularité 7 ans après la négativation. Le délai médian des rechutes fut de 4,4 ans, et ne fut pas influencé par la régularité au traitement.RESUMEN
De los 47,068 pacientes registrados en el Centro Leprológico Polambakkam entre 1955 y 1982, se seleccionaron 1886 casos que independientemente de su clasificación al ingreso, fueron bacteriológicamente positivos y posteriormente se tornaron bacteriológicamente negativos. Después de un período de seguimiento promedio de 10 años se observaron 243 recaídas, lo que equivale a una frecuencia global de recaída de 12.8 por persona-años de observación, y una probabilidad acumulativa de recaída del 18.9%. Se encontró que la frecuencia de recaída dependió de la regularidad del tratamiento durante los periodos bacteriológicamente positivos y los negativos; una regularidad mayor del 75% en el período de positividad bacteriológica resultó ser particularmente importante. Los resultados no mostraron evidencias de que las recaídas ocurridas después de 3 años de negatividad pudieran deberse a reinfecciones y que la frecuencia de recaída estuviera afectado aún por la regularidad del tratamiento 7 años después de la negativización. El retardo promedio en la aparición de recaídas fue de 4.4 años y no estuvo afectado por la regularidad del tratamiento.Multidrug therapy (MDT) for multibacillary patients is now imperatively recommended and largely applied. Dapsone monotherapy is, however, still used on a large scale in many countries in the world where insufficient resources and poor accessibility of remote areas delay the introduction of MDT. Health officers who are in charge of leprosy control programs in such conditions are still concerned with knowing what the risk of relapse is for the multibacillary patients, and how long dapsone monotherapy should be continued after negativation. Following findings showing that these cases frequently relapsed after treatment was stopped, the recommendation was expressed that they should be treated for at least 10 years after negativation 4). The burden of such a policy on health services, in terms of maintaining expensive health care facilities and ambulatory services for long-term patients, has, however, led to a discussion of whether these patients really benefit from continuous treatment. Baseline data on relapses occurring in a large population of multibacillary cases treated with dapsone (DDS), and followed over a long period of time, are therefore necessary to give valid indications regarding the long-term surveillance of these patients.
The relapse rate observed after completion of treatment is also identified as an important indicator to measure the efficacy of MDT regimens (15). In this scope, it is interesting to discuss whether data on relapses observed with dapsone monotherapy could be used as references for comparison.
This paper is, therefore, an attempt to study the rate and time distribution of relapses occurring in a large population of multibacillary patients treated with dapsone, and to evaluate the effect of different levels of regularity of treatment on these indicators.
MATERIALS AND METHODS
Of the 47,068 patients registered in the Polambakkam Leprosy Center (South India) between 1955 and 1982, the present study includes only those who showed a bacterial index (BI) of > 2+ on the Ridley scale (5), at any time during this period, and reached bacteriological negativity. Bacteriological negativity was defined by two consecutive years with a BI equal to 0, the year of negativation being taken as the second of these two years. These criteria were chosen in order to ascertain both the multibacillary status, whatever the classification at the registration, and the smear negativity, by excluding the cases who were found to present a 131 equal to 0 in a single year. A total of 1886 cases met the above criteria. The definition of relapse was also based on a bacteriological criterion, i.e., the presence of two consecutive years with a BI > 1 + . This criterion was satisfied by 254 cases, and from these, 11 had an incomplete follow-up period, and were found bacteriologically positive the same year they resumed attendance at clinics. Since we had no indications that they resumed treatment the same year of their positivation, they were removed from calculation in order to avoid ovcrestimation of the delay of relapses and of the denominator of the relapse rate. Their observation time until their disappearance was, however, taken into account. A total of 243 relapses were thus considered. The remaining 1632 patients were either lost to observation because they died or disappeared or reached the end of the year 1982 (when the study period stopped) without relapsing. To take into account their follow-up time until withdrawal, the relapse experience of the population was studied by constructing a Kaplan-Meier life table (7) with censored observations. Three indicators were calculated: a) The relapse rate was computed by dividing the total number of relapses by the total number of person-years of observation after bacteriological negativation. The significance test for the ratio of two rates with person-time denominator was computed using a logarithmic transformation, to adjust for the asymmetric sampling distribution of the rate ratio (11). b) The cumulative probability of relapse from year 0 to year / was defined as the complement of the probability to "survive" (not to relapse) longer than year c) The delay of relapses was taken as the difference between the first year of relapse and the second year of negativity. In fact, since patients were enrolled only if they presented bacteriological negativity for two consecutive years, they were not at risk of relapsing during the second of these two years. The median delay was calculated as the moment at which 50% of the relapses had occurred. The Median Test (3) was used to examine whether two samples came from populations having the same median.
During the study period, the treatment scheme was dapsone monotherapy for all multibacillary patients without discontinuity, even after bacteriological negativation. As described by Vellut (12), the method of treatment was to try to reach a total dose of 400 mg to 600 mg of DDS per week. Regularity was defined as the number of attended sessions of treatment divided by the number of organized sessions. Patients were assumed to have ingested all of the tablets they received. In a survey carried out by Vellut in 1966, the number of tablets that were not ingested was estimated to represent less than 20% of the total of the tablets received (unpublished data).
Two periods of treatment were considered: the smear-positive period, during which patients presented a BI > 1, and the smear-negative period, when the BI was equal to 0. During this last period, the bacteriological status was assessed by one routine bacteriological examination per year. The "follow-up" period refers to the period of observation starting after negativation.
No inoculation of the mouse foot pad was performed to test for the presence of DDS resistant bacilli.
RESULTS
The 243 relapses were observed in a total of 18,941 person-years of observation, giving a crude relapse rate of 12.8 per thousand person-years, with a 95% confidence interval of 11.2 to 14.4 per thousand. After a maximum of 25 years of observation, the cumulative probability of relapse came to 18.9%. Figure 1 illustrates the progression over time of the cumulative probability of relapses on a logarithmic scale. No relapses were observed during the first year after negativation (i.e., before completion of the first year of follow-up). As can be seen from the slope of the curve, the relapse rate is thereafter maximum during the second year of observation, and then decreases over time up to the seventh year when it becomes roughly constant. The last relapse was observed during the 21st year.
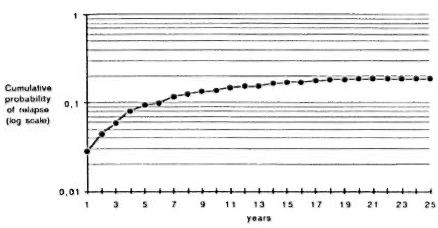
Fig. 1. Cumulative probability of relapse by year of follow-up.
In order to study the effect of regularity of treatment in smear-positive and smear negative periods, the data have been divided into four groups of regularity in each period: < 25%, from 25% to 49%, from 50% to 74%, and > 75%. Figure 2 shows that patients who had a regularity > 75% in the smear-positive period achieved a lower probability of relapse than the other three groups. On the other hand, Figure 3 indicates that each group of regularity in the smear-negative period led to a different level of cumulative probability of relapse.
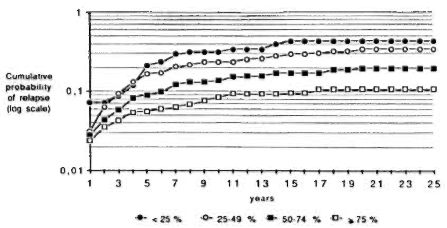
Fig. 2. Cumulative probability of relapse by year of follow-up and by regularity status during smear-positive period.
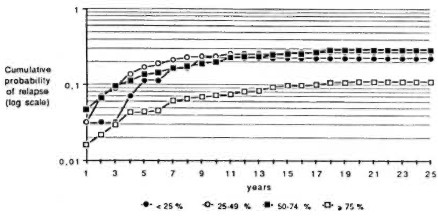
Fig. 3. Cumulative probability of relapse by year of follow-up and by regularity status during smear-negative period.
Therefore, it seemed interesting to study the effect of the different levels of regularity during both periods. For this purpose, two levels ofregularity have been chosen in each period, < 75% and > 75%. Figure 4 illustrates that the highest cumulative probability of relapse is observed for patients irregular in both periods, and the lowest for those regular in the two periods. It is noteworthy that the two groups experiencing the lower cumulative probabilities of relapse are those with a high regularity during the smear-positive period. The corresponding rates are presented in Table 1. They range from 4.6 per thousand, for patients regular in both periods, to 25.0 per thousand for irregular cases. It shows that an estimation of the relapse risk has to take into account the regularity during the two periods simultaneously. Again, the lower rates are found for the two groups with a high regularity during the smear-positive period.
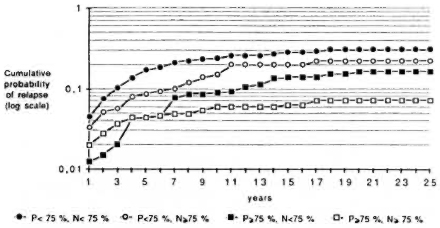
Fig. 4. Cumulative probability of relapse, by regularity status during smear-positive (P) and smear-negative (N) periods.
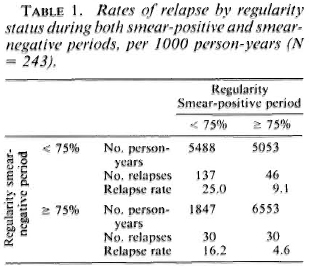
It also seemed interesting to study whether regularity at treatment during one period or the other had a similar effect on relapse rates in different time intervals of follow-up. Table 2 presents relapse rates by regularity status during the smear-positive period in three intervals: from 0 to 3 years, from 4 to 6 years, and at 7 years and more. Highly significant differences (p < 0.01) are found in each of these three intervals, the relapse rates being higher for patients who are irregular in treatment.
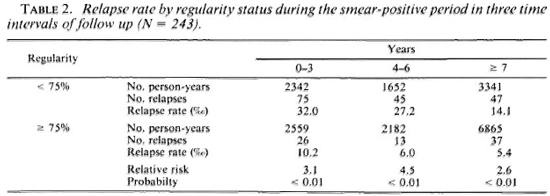
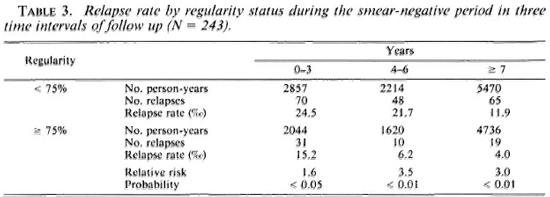
Table 3 indicates that similar results are observed when regularity during the smear-negative period is considered: a statistically significant difference (p < 0.05) in the 0-3 year interval and a highly statistically significant difference (p < 0.01) in the two other intervals are found between the rates.
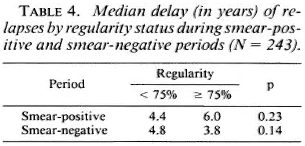
The median delay of relapses turned out to be 4.4 years. It was computed for the two groups of regularity in each period and ranged from 3.8 years to 6.0 years, as presented in Table 4. The Median Test showed no statistically significant differences at the 5% level between groups for the two periods.
DISCUSSION
Results on relapses that could be used for inter-study comparisons are scarce in the literature. One reason is the small number of patients surveyed and/or the short duration of follow-up after negativation. A second reason is the lack of standardization in the research methodology. One example among others is the definition of the "relapse rate," which is taken either as a simple proportion (expressed as the number of patients who relapsed divided by the number of patients who were negative at the beginning of the study period) or as a "true" rate with the sum of time-periods of observation as the denominator (expressed in person-years of observation).
The present study retrospectively surveys a total of 1886 multibacillary patients who were followed-up for 1 to 25 years after negativation (an average of 10 years) and who received continuous treatment with dapsone monotherapy. The selection criteria were chosen in order to ascertain with good specificity the multibacillary status, the smear negativity, and the bacteriological relapse. Moreover, they are similar to criteria used in other studies (1,8,12), which enables comparisons. A crude relapse rate of 1.28% person-years was observed, ranging from 0.46% to 2.5%, according to the regularity status. A further analysis indicated a relapse rate of 5.0% person-years for the most irregular cases, with a regularity during the smear-positive period of < 75% and a regularity during the smcar-ncgativc period of < 25%. These results can be compared to those described in other studies: a) Erickson (4) reported a relapse rate of 1.7% person-years among 22 patients continuing treatment after negativation, and of24.4% among the 11 who stopped treatment, b) Ncclan (8) observed a relapse rate of2.2% person-years in 454 multibacillary patients followed up for 1 to 9 years, ranging from 1.5% for the cases with a regularity > 50% in the smear-negative period to 3.5% for the cases with a regularity of < 50%. c) Among 362 lepromatous patients treated for about 20 years with dapsone monotherapy, and subsequently released from control, Waters, et al. (13) found a relapse rate of 1.04% person-years of observation during a follow-up period of 8 to 9 years; and d) Reconstruction of the data presented by Almeida, et al. (1) on 1008 patients followed up for about 9 years indicates a crude relapse rate of 2.2% person-years, ranging from 1.5% to 3.8% according to the treatment regularity status.
Since our data suggest that relapse rates depend largely on the regularity of treatment status during both bacteriologically positive and negative periods, it is therefore possible that, besides differences in methodologies, different results between studies could be explained by differences in the regularity status of the studied populations, or by different cut-off points chosen to differentiate "regular" and "irregular" cases. The data presented here also suggest that regularity during the smear-positive period has a greater effect on relapse rates than regularity during the smear-negative period, and that a regularity > 75% in the first period is particularly important.
The duration of treatment after negativation is an issue of continuing interest (8,13). Results published by Neelan (8) showed that after 3 years of negativity, the relapse rates were similar in two groups of patients with different levels of regularity in the smear-negative period. This suggested that treatment would be unnecessary beyond 3 years after negativation. The relapse risk was, however, still high from 4 to 6 years after negativation, and no data were available on relapses occurring at that time if the treatment was stopped. He, therefore, suggested that it is only after 7 years that patients could be removed from treatment. The results presented here do not confirm this conclusion, since differences in relapse rates are still observed after 7 years of negativity. On the contrary, they support the conclusions of some previous studies (4,10,12) which favored long-term treatment.
Another suggestion was expressed by Almeida, et al. (1) They found that after 3 years of negativity the relapse risk was independent of regularity during the smear-positive period. It was, therefore, argued that relapses occurring beyond the first 3 years of negativation could be reinfections instead of relapses. Again, this hypothesis is not supported by our data, since significant differences are found even after 7 years in the direction of higher relapse rates for irregular patients.
The cumulative probability of relapse came to 18.9%. With a life-table method, this indicator takes into account the number of cases who are left in each time interval after removing those who relapsed or were withdrawn. It is thus greater than a simple proportion (243/1886 = 12.9%).
Very few data are found in the literature regarding the median delay of relapses calculated in a large population of patients treated with dapsone and followed up for many years after negativation. Inter-study comparisons are also to be taken with great caution, since the median delay is particularly affected by the research methodology, i.e., definitions of negativation and of relapse. It is also an indicator whose accuracy depends on the length of the observation period and on the sample size. In spite of this, it has been used by some authors for the determination of the optimum duration of follow-up after completion of treatment, even when calculated on a small number of relapses (6,9). The following example illustrates the difficulty of interpreting the median delay: In a total of 576 multibacillary patients registered from 1955 to 1958 and observed up to 1968, Vellut (12) found a median delay of 5.5 years. Our results relate to the same source population, with enrollment extended up to 1982, and concern 1886 multibacillary patients observed for an average of 10 years after negativation. The median delay of relapses turned out to be 4.4 years, and was not significantly affected by the regularity status. The difference between the two studies is best explained by the variations in the methodology and by the differences in the number of patients surveyed and the number of years of observation.
Potential limitations of this study are worth mentioning. Firstly, from all of the multibacillary cases registered in Polambakkam from 1955 to 1982, only those who were known to reach bacteriological negativity were enrolled in the study. A selection bias could have occurred, since positive patients who interrupted treatment after awhile could have become negative and presented a different relapse experience. Secondly, the negative cases who disappeared may be those with a low regularity status during smear-positive and/or smear-negative periods. A comparison of relapse rates in relation to groups of treatment regularity could be biased by not taking into account the most irregular cases. Lastly, an underlying assumption of the life-table analysis in a follow-up study is that the probabilities of undergoing an event remain reasonably constant over time (2). This implies that patients entering the study in different calendar years would be submitted to the same risks of relapse. This assumption may not be verified if the treatment scheme is modified or if treatment regularity varies in different periods of time, whatever the reason.
Could these results be used as references with which to compare relapses observed after MDT? Possibly yes, when the cumulative probability and the rate of relapse are considered and if the same methodology is applied. The appropriateness of the use of the median delay of relapses for the same purpose would, however, be doubtful. Differences in the biological effect between the two treatments (bacteriostatic for dapsone, bactericidal for rifampin), as well as the very low relapse rates observed so far with MDT, would make the validity of such a comparison questionable. The median delay of relapses observed after MDT could be even longer than with dapsone monotherapy, since these "relapses" would occur theoretically in patients freed of all viable bacilli and would therefore more often be reinfections.
The generalizability of this study for MDT lies more in its methods than in its results. MDT is assessed by evaluating the efficacy of various regimens, and inter-study comparisons will be useful only with reference to similar methodologies. This study presents a valid and simple method for describing the relapse experience in a population that could be used for the assessment of MDT regimens. It includes the construction of a Kaplan-Meier life table, the calculation of cumulative probabilities of relapse, and the computation of relapse rates using the number of person-years of observation as the denominator.
Recommendations for the surveillance of multibacillary leprosy have to take into account that dapsone monotherapy is still used on a large scale when local conditions delay the implementation of MDT. In conclusion, regarding these patients, this study suggests: a) The crude relapse rate and the cumulative probability of relapse are affected by regularity status during both smear-positive and smear-negative periods, and an estimation of relapse risk has to take into account attendance during both periods, b) Regularity in treatment during the smear-positive period is more important than regularity during the smear-negative period, c) A regularity > 75% in the smear-positive period decreases significantly the relapse rate, even after 7 years of follow-up. There are, therefore, no indications that relapses occurring from that time on are reinfections, d) Different levels of regularity during the smear-negative period lead to different relapse rates, and a regularity > 75% decreases significantly the rates, even after 7 years. In Polambakkam, the rate was still high and it was better to continue dapsone monotherapy. More generally, the decision to interrupt the treatment of negative cases should be made assessing the risk of relapse on the basis of the observed rates, and taking into account the cost of maintaining drug delivery services; and e) The median delay of relapses turned out to be 4.4 years, and this was not significantly affected by regularity of treatment during cither the smear-positive or smear-negative period.
Acknowledgments. This study was supported by the Amici di Raoul Follereau, Association Française Raoul Follercau, Damien Foundation Belgium, Fondation Luxembourgeoise Raoul Follereau, and Le Secours aux Lépreux, Canada (Member-Associations of ILEP).
REFERENCES
1. ALMEIDA, J. G., JESUDASAN, K., CHRISTIAN, M. and CHACKO, C. J. G. Relapse rate in lepromatous leprosy according to treatment regularity. Int. J. Lepr. 54(1986)16-20.
2. ARMITAGE, P. and BERRY, G. Statistical Methods in Medical Research. 2nd ed. Oxford: Blackwell Scientific Publications, 1987, p. 427.
3. CONOVER, W. J. Practical Nonparametric Statistics. 2nd ed. New York: John Wiley & Sons, 1980, pp. 171-176.
4. ERICKSON, P. T. Relapse following apparent arrest ofleprosy by sulfone therapy. Int. J. Lepr. 19(1951)63-74.
5. HASTINGS, R. C. Leprosy. Edinburgh: Churchill Livingstone, 1985, p. 45.
6. JANSSENS, L., BOURLAND, J., GROENEN, G., NOLLET, E. and PATTYN, S. R. Incubation time of relapses in multibacillary leprosy. In: XII International Leprosy Congress Proceedings, New Delhi, February 20-25, 1984. Desikan, K. V., ed. New Delhi: PRINTAID, n.d., pp. 17-18.
7. LEE, E. T. Statistical Methods for Survival Data Analysis. Belmont, California: Lifetime Learning Publications, 1980, pp. 75-87.
8. NEELAN, P. N. Relapse in lepromatous leprosy under sulphone treatment. Lepr. India 44(1972)98-102.
9. PATTYN, S. R. Incubation time of relapses after treatment of paucibacillary leprosy. Lepr. Rev. 55(1984)115-120.
10. QUAGLIATO, R., BECHELLI, L. M. and MARQUES, R. M. Bacterial negativity and reactivation (relapse) of lepromatous outpatients under sulfone treatment. Int. J. Lepr. 38(1970)250-263.
11. ROTHMAN, K. J. Modern Epidemiology. Boston: Little Brown and Company, 1986, pp. 170-172.
12. VELLUT, C. A follow-up study of over 10 years of lepromatous leprosy with reference to response to specific treatment and occurrence of relapse. Lepr. India 41(1969)276-281.
13. WATERS, M. F. R., REES, R. J. W., LAING, A. B. G., FAH, K. K., MEADE, T. W., PARIKSHAH, N. and NORTH, W. R. S. The rate of relapse in lepromatous leprosy following completion of twenty years of supervised sulphone therapy. Lepr. Rev. 57(1986)101-109.
14. WHO EXPERT COMMITTEE ON LEPROSY. Fourth report. Geneva: World Health Organization, 1970. Tech. Rep. Ser. 459.
15. WHO STUDY GROUP. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
1. M.D., Medical Officer; Epidemiology Unit, Catholic University of Louvain, Brussels, Belgium.
2. M.D., Medical Officer, Epidemiology Unit, Catholic University of Louvain, Brussels, Belgium.
3. M.D., Medical Officer, Hemerijekx Government Leprosy Center, Polambakkam, Tamil Nadu, India.
Reprint requests to: Dr. E. Declercq, EPID 30.34, School of Public Health, Catholic University of Louvain, 30 Clos Chapelle-aux-Champs, 1200 Brussels, Belgium.
Received for publication on 29 November 1988.
Accepted for publication in revised form on 8 March 1989.