- Volume 57 , Number 3
- Page: 607–14
Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin
ABSTRACT
Among 39 strains of Mycobacterium leprae isolated f rom patients with multibacillary leprosy who relapsed after treatment with rifampin (RMP), 22 strains were resistant to RMP and 17 were susceptible. All of the RMP-resistant strains were recovered f rom patients who had been treated with more than 50 doses of RMP, usually given as monotherapy. RMP-susceptible strains were recovered f rom only six patients who had received more than 50 doses of RMP, and f rom 11 patients who had received no more than seven doses. The median time to relapse after the beginning of RMP therapy was 9 years (range 1-12 years) among the patients harboring RMP-resistant strains of M. leprae, and the median time to relapse after discontinuation of RMP treatment was 7 years (range 1-11 years) among the patients harboring RMP-susceptible strains. These data suggest that monotherapy with more than a few doses of RMP can be responsible for the emergence of RMP-resistant strains of M. leprae, thus emphasizing the need to employ RMP only in combination with other effective drugs in the chemotherapy of multibacillary leprosy.RÉSUMÉ
Parmi 39 souches de Mycobacterium leprae isolées chez des malades atteints de lèpre multibacillaire, ayant récidivé après un traitement par la rifampine (RMP), 22 souches étaient résistantes à cet antibiotique et 17 étaient encore susceptibles. Toutes les souches encore résistantes à la rifampine ont été obtenues chez des malades qui avaient été traités par plus de 50 doses du produit, généralement administré en monothérapic. Des souches susceptibles à la rifampine n'ont été rccuicllics que chez 6 malades qui avaient reçu plus de 50 doses du produit, et chez 11 malades qui n'avaient reçu que 7 doses. La médiane de la durée entre le début de la thérapie par la rifampine et la résistance était de 9 ans (s'étendant de 1 à 12 ans) parmi les malades porteurs de souches de M. leprae résistantes à la RMP; la médiane de la durée s'étendant entre le cessation du traitement par la rifampine et la récidive était de 7 ans (de 1 à 11 ans) parmi les malades porteurs de souches susceptibles à ce produit. Ces données suggèrent que la monothérapie, lorsqu'elle dépasse quelques doses de rifampine, pourrait être responsable de l'apparition de souches de M. leprae résistantes au médicament. Ceci souligne la nécessité, en chimiothérapie de la lèpre multibacillaire d'employer la rifampine uniquement en combinaison avec d'autres médicaments efficaces.RESUMEN
De 39 cepas de Mycobacterium leprae aisladas de pacientes con lepra multibacilar que recayeron después de tratamientocon rifampicina(RMP), 22 cepas fueron resistentes a RMP y 17 fueron sensibles. Todas las cepas RM P-resistentes fueron aisladas de pacientes que habían sido tratados con más de 50 dosis de RMP usualmente administrada como monoterapia. Las cepas susceptibles a RMP se recuperaron sólo de 6 pacientes que habían recibido más de 50 dosis de la droga y de 11 pacientes que habían recibido no más de 7 dosis. El tiempo promedio de recaída después de iniciar la terapia con RMP fue de 9 anos (1-12 anos) en los pacientes con las cepas de M. leprae resistentes a la RMP; el tiempo medio de recaída después de descontinuar el tratamiento con RMP fue de 7 anos (1-11 anos) en los pacientes con M. leprae susceptibles a RMP. Estos datos sugieren que la monoterapia con varias dosis de RMP puede ser responsable de la emergencia de cepas del M. leprae resistentes a la droga y enfatizan la necesidad de emplear RMP sólo en combinación con otras drogas efectivas en la quimioterapia de la lepra multibacilar.In 1981, a World Health Organization (WHO) Study Group recommended (20) a standard regimen for the chemotherapy of multibacillary leprosy, based on the combination of rifampin (RMP) with dapsone (DDS) and clofazimine (CLO). During the years preceding 1981, however, nonstandard RMP-containing regimens were employed in many areas. RMP had been prescribed in a variety of dosages and rhythms of administration and for various durations, either as monotherapy or in combination with one drug-DDS or a sulfonamide - that had already been employed for a long period of time. In most instances, RMP was prescribed only after the patient had suffered a relapse, after having been treated with DDS or a sulfonamide as monotherapy and therefore presumed to harbor dapsoneresistant Mycobacterium leprae. The increasing prevalence of DDS resistance as a consequence of DDS monotherapy (3-6) suggested that monotherapy with RMP would result in a high relapse rate and the emergence of RMP-resistant M. leprae. A number of such cases have been reported by Jacobson and Hastings (5) and by Guelpa-Lauras, et al.(4), justifying this concern.
Including the cases already described by Guelpa-Lauras and her coworkers (4), 39 strains of M. leprae have been isolated from patients with multibacillary leprosy, in Guadeloupe, Martinique, New Caledonia, Senegal, and metropolitan France, who relapsed after some course of treatment by RMP. The purpose of this paper is to describe the studies of susceptibility to RMP and to examine the relationships among treatment before relapse, susceptibility to RMP, and the length of the interval between treatment and relapse.
MATERIALS AND METHODS
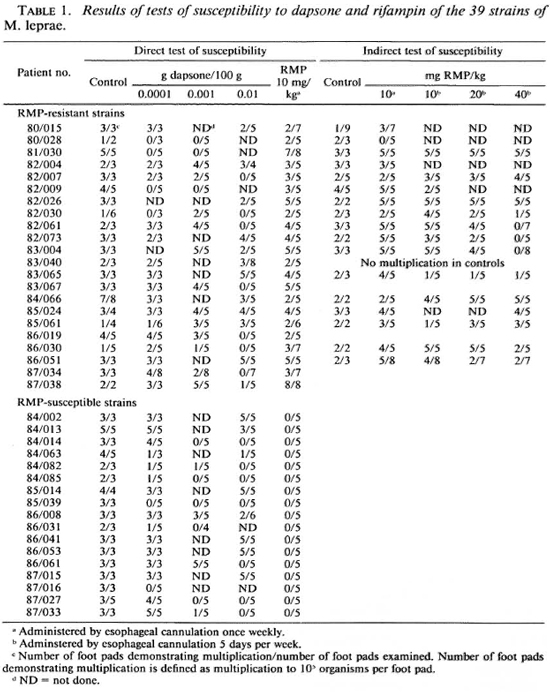
Since RMP first became available, a total of 404 patients, 150 from Guadeloupe (1), 71 from Martinique, 113 from New Caledonia and 70 from Senegal (7,8), were known to have been treated, mainly before the year 1980, by some nonstandard RMP regimen, varying from a single dose of 1500 mg to daily treatment for several years. Except for those from Senegal, the patients had been followed and examined regularly every 6 months. In the event of relapse, defined as the development of new skin lesions containing a large number of acid-fast bacilli (AFB) and histopathological characteristics of active lepromatous leprosy, a skin lesion was biopsied. The biopsy specimen was sent to Paris for mouse inoculation to determine the susceptibility of the patient's organisms to DDS and RMP. M. leprae were isolated from 35 of these 404 patients, 3 from Guadeloupe, 17 from Martinique, 4 from New Caledonia, and 11 from Senegal. In addition to these 35 strains, four strains were isolated from patients treated by nonstandard RMP-containing regimens in metropolitan France. The skin-biopsy specimens were air-shipped to Paris in thermocontainers (Nunc, Roskilde, Denmark). The median interval between performance of the biopsy and receipt of the specimen in Paris was 2 days (range 1-5 days). Upon arrival in Paris, the specimens were weighed and minced, the homogenatcs were suspended in Hanks' balanced salt solution, and the AFB were enumerated by Shcpard's method (14,16) in 20 oil-immersion fields in each of two 10 μl drops. The organisms were then diluted and 5000, contained in a volume of 30 μl were inoculated into the left hind-foot pads of four-week-old, outbred Swiss female mice. For each specimen, 42 mice were inoculated-10 untreated controls, 3 groups of 8 mice each treated with DDS incorporated into the diet in a concentration of 0.0001, 0.001 or 0.01 g per 100 g diet, and a fourth group of 8 mice administered RMP in a dosage of 10 mg per kg body weight once weekly by esophageal cannula ("gavage") (13). The treatment of the mice was begun on the day of inoculation, and continued until the mice were harvested for enumeration of the AFB (16). Beginning 7 months after inoculation, harvests of AFB were performed from the foot pads of three control mice at intervals of 2 ½ months. When the AFB were noted to have multiplied in control mice to > 105 per foot pad, usually between 9 and 12 months after inoculation, harvests of AFB were carried out from the foot pads of the treated mice. Resistance to DDS or RMP was defined as multiplication to > 105 organisms per foot pad in at least one treated mouse (3,6).
To confirm the results of the direct test of drug susceptibility and to determine the level of resistance to RMP, RMP-resistant strains of M. leprae were subinoculated into mice that were subsequently administered RMP by gavage in a weekly dose of 10 mg per kg, or in a daily dose of 10, 20, or 40 mg per kg 5 days per week.
Among the patients relapsing with RMP-resistant M. leprae, the "incubation period" of relapse has been taken to be the interval between beginning treatment by RMP and relapse, the time during which RMP-resistant'mutants were selected. Among those patients relapsing with M. leprae susceptible to RMP, the incubation period of relapse has been assumed to be the interval between cessation of treatment by RMP and relapse, the time during which regrowth of viable RMP-susccptiblc organisms occurred.
Data were analyzed by Pearson's chisquarcd test and by the technique of exploratory data-analysis (18).
RESULTS
Susceptibility to DDS and RMP. The results of the tests of susceptibility to DDS and RMP of the 39 strains of M. leprae isolated in mice from patients who relapsed after some treatment by RMP are summarized in Table 1. Twenty-two (56.4%) of the 39 strains were found to be resistant to RMP as a result of the direct test performed in the course of primary isolation of the organisms. Among the 22 strains found resistant to RMP, 19 (90.5%) out of 21 strains in which DDS susceptibility could be measured were resistant to DDS - 12 were fully resistant to DDS, 7 exhibited an intermediate degree of resistance, and only 2 strains were fully susceptible; the susceptibility to DDS of one strain (80/028) could not be determined because of the small number of harvests performed from control mice. Among the 17 strains found susceptible to RMP, 15 (88.2%) were resistant to DDS- 8 were fully resistant to DDS, 3 exhibited an intermediate degree of resistance, 4 were of low-dcgrcc resistance, and 2 strains were fully susceptible to DDS. The distribution among RMP-susceptible strains of the various degrees of resistance to DDS does not differ significantly from that among the RMP-resistant strains. The susceptibility to RMP of the 22 RMP-resistant strains was retested in the course of passage of the strains to new mice; results are available for 17 of the 22 strains. The organisms of one strain (80/028) did not multiply in RMP-treated mice. Resistance to RMP of the remaining 16 strains was confirmed. Of the 13 strains tested for susceptibility to RMP administered 5 days per week, 10 multiplied in mice administered the drug in a dosage of 40 mg per kg body weight and the remaining 3 strains in mice administered 20 mg RMP per kg. This uniformity among strains and the failure of strains to demonstrate a range of susceptibility is consistent with the hypothesis (19) that resistance to RMP results from a single-step mutation.
Treatment histories of the patients. The found to harbor strains of M. leprae resistreatment histories of individual patients tant to RMP had been treated with RMP before the administration of RMP are sumadministered daily for at least 5 months; 16 marized in Table 2. It is evident that a great had received RMP by some regimen for at majority of the patients had been treated for least 12 months, 2 of the patients having a long time with DDS/sulfonamide monobeen treated for 72 months. One of the 22 therapy. Twenty-one of the 22 patients patients (86/051) had received RMP in 1976 in a dosage of 900 mg once weekly as monotherapy for 3 months, followed by 50 mg DDS daily as monotherapy for 5 months, after which he failed to continue treatment. Seven years later, his multibacillary leprosy was found to have relapsed, both clinically and histopathologically; he was treated for only 40 days with 600 mg RMP, 500 mg ethionamide, 100 mg CLO, and 100 mg DDS, all drugs administered daily, after which he again failed to continue treatment. To 18 of the 19 patients whose strains of M. leprae were found to be resistant to DDS, RMP was administered either as monotherapy or in combination only with DDS or a sulfonamide. The only patient to be treated with RMP in combination with a companion drug not DDS or a sulfonamide is patient 86/051. Although the M. leprae of two patients were fully susceptible to DDS, one (81/030) is known to have been treated with RMP as monotherapy, and the second (82/ 009) may also have been so treated. In the case of only one additional patient (80/028) may RMP have been administered in combination with an effective companion drug; this patient's M. leprae were not demonstrated to be resistant to DDS, and DDS was administered during the entire 47 month course of treatment with 600 mg RMP daily. Thus, 20 of the 22 patients whose strains of M. leprae have been found resistant to RMP were treated with RMP administered essentially as monotherapy.
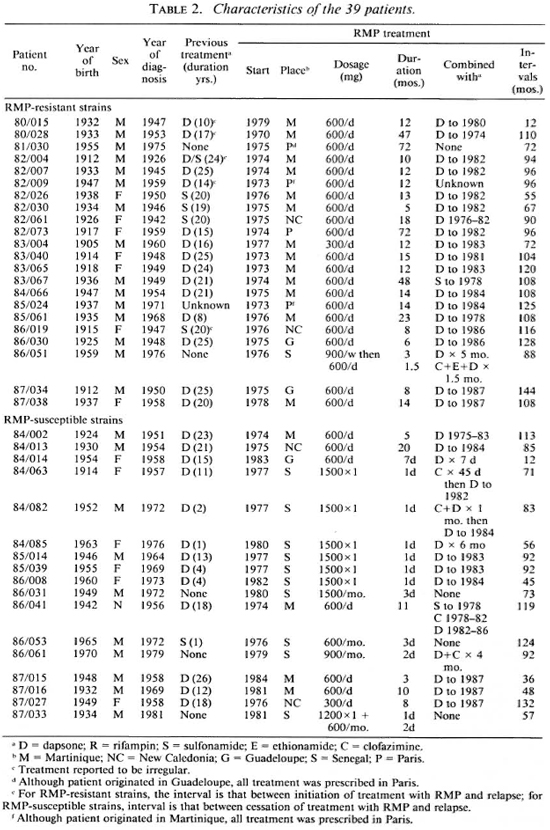
Similarly, all but one or two of the 17 patients found to harbor RMP-susceptible organisms may be regarded as having'been treated with RMP as monotherapy. Six of these patients had been treated with RMP daily for from 3 to 20 months: RMP was administered to one patient (84/002) without a companion drug and 5 were treated simultaneously with DDS or a sulfonamide; however, the M. leprae of 5 of these 6 patients were resistant to DDS, all but one strain demonstrating a high degree of resistance. The 11 remaining patients had been treated with only a few doses of RMP-6 patients, 5 of whom harbored DDS-resistant strains of M. leprae, had been administered single 1500 mg doses of RMP simultaneously with DDS, and 2 had also received brief courses of CLO; 5 additional patients, all of whose M. leprae strains exhibited resistance to DDS, had been given from 2 to 7 doses of RMP, and only 2 of them had been treated with companion drugs (DDS in one case, and DDS plus CLO in the other).
None of the 22 patients harboring RMP-resistant strains of M. leprae had been treated with fewer than 50 doses of the drug; whereas only 6 of the 17 harboring susceptible strains had received this many doses of RMP. This difference among the patients is significant (p < 0.00001).
Incubation period of relapse. Among the 22 patients whose strains of M. leprae were found resistant to RMP, the median interval between initiation of treatment with RMP and relapse was 106 months (range 12-144 months). There was no relationship between duration of RMP treatment and duration of the interval between initiation of treatment and relapse (p = 0.50). Among the 17 patients whose strains of M. leprae were found susceptible to RMP, the median interval between cessation of treatment with RMP and relapse was 83 months (range 12-132 months); in this case also, no relationship could be discerned between duration of treatment and duration of the interval between initiation of treatment and relapse (p > 0.24). Finally, the median interval between initiation of treatment and relapse for those 22 patients whose strains of M. leprae were resistant to RMP was not different from that for the 17 patients whose organisms were susceptible to RMP (p > 0.05). To compare these two periods, the duration of treatment by RMP was added to the interval between cessation of treatment by RMP and relapses for those patients who relapsed with RMP-susceptible organisms.
DISCUSSION
Among 39 patients with multibacillary leprosy who relapsed after some period of treatment with RMP, almost always administered as monotherapy, 22 were found to harbor strains of M. leprae resistant to RMP. Resistance was confirmed by a second test for all but one of these 22 strains. Thus, there can be no doubt about the reality of resistance to RMP. Moreover, virtually all of the strains were found to be resistant to a high level of RMP, supporting the hypothesis that resistance to RMP results from a single-step mutation.
A remarkable finding was that all 22 of the patients who harbored RMP-resistant strains of M. leprae had received more than 50 doses of the drug; whereas only 6 of the 17 patients whose strains of M. leprae were susceptible to RMP had received this many doses. In addition, it was noted that almost all of the M. leprae of those patients harboring strains resistant to RMP were also resistant to DDS; thus, despite concomitant treatment with DDS or a sulfonamide, RMP was being administered to these patients essentially as monotherapy. Therefore, the three conditions required for selection of a drug-resistant mutant, i.e., large microbial populations, numerous doses of the selecting antimicrobial agent and the absence of an effective companion drug, were all fulfilled in these patients.
Although most of the strains of M. leprae isolated from the 17 patients found to harbor organisms susceptible to RMP were resistant to DDS, and RMP was administered essentially as monotherapy, most of these patients received only small quantities of RMP. From this, one may infer that RMP-containing regimens that deliver a limited number of doses of the drug (7-8-12) are less actively bactericidal than are regimens that deliver numerous doses. This analysis confirms the need for RMP-containing regimens of long duration, an example of which is the WHO Study Group regimen (20).
On the other hand, implicit in our data is the fact that not all patients treated with RMP as monotherapy for long periods of time relapse with M. leprae resistant to RMP. In fact, 28 of the 39 patients had received more than 50 doses of RMP, and only 22 of them were found to harbor RMP resistant organisms. Similar findings have been observed after long-term monotherapy with DDS (6). That six patients relapsed with RMP-susceptible organisms after a course of RMP as monotherapy of sufficient duration to have selected the RMP-resistant mutants in most of the patients cannot be explained by the absence of such mutants; at the time of the first relapse, when RMP was first prescribed, these patients were observed to have heavily positive slit-skin smears. The most likely explanation is that the RMP-susccptible organisms did not experience a burst of multiplication, perhaps because they were present in a dormant or persisting state (17) that rendered them phenotypically resistant to RMP.
A subject of considerable interest is the incubation time of relapse. In this study, the median duration of the incubation period was 9 years for patients relapsing with RMP resistant strains of M. leprae. If the number of spontaneously occurring RMP-resistant mutants is no greater than one in 109-1010 viable M. leprae (2,15), then a median duration of 9 years was much longer than required for 103 organisms to multiply to 109 and cause relapse. Because the doubling time of M. leprae is of the order of 2 weeks (14), only 1 year should have been required for 103 RMP-resistant mutants to multiply to 109. It is apparent, therefore, that the RMP resistant mutants did not begin to multiply immediately after RMP monotherapy was instituted, nor did they continue to multiply regularly after RMP was withdrawn. Rather, it appears more likely that the organisms experienced bursts of multiplication followed by long periods of persistence. The evidence for the bursts of multiplication is that selection of the RMP-resistant mutants occurred in 22 of 28 patients; evidence for long periods of persistence is the long incubation time of relapse.
The median incubation time for patients relapsing with RMP-susceptible strains was 7 years, not significantly shorter than the incubation time among patients harboring RMP-resistant strains of M. leprae. The long interval between cessation of RMP administration and relapse suggests that, after cessation of treatment, the organisms remained in a persisting state for long periods of time. That the incubation time of relapse was similar in both groups of patients suggests that the population of RMP-susceptiblc persisting M. leprae may be of the same size as that of the population of RMP-resistant mutants. Such an hypothesis is consistent with current concepts (2) and available data (9,17).
Finally, the broad range of incubation times suggests large differences in the ability to contain small populations of M. leprae from patient to patient. If this be so, then some patients may not relapse until many years after treatment has been completed. Although it has been suggested (10-12) that 50% of the relapses in multibacillary leprosy should appear within 3 years of stopping treatment, our data suggest that the majority of relapses may occur only much later. Therefore, a long period of follow up is required before a multibacillary patient may be regarded as cured.
Acknowledgments. The writers thank Evelyne Perani, Corinne Bcoletto and Delphine Dufosset for their dedicated work, and are indebted to Professor Louis Levy and Dr. Ji Baohong, Secretary of THELEP, WHO, Geneva, for providing help and advice in the preparation of this manuscript.
REFERENCES
1. CARTEL, J.-L., NAUDILLON, Y., REMY, J.-C. and GROSSET, J. H. Contribution of relapses to total infection sources of leprosy in Guadeloupe. Lepr. Rev. 58(1987)339-348.
2. GROSSET, J. Recent developments in the field of multidrug therapy. Lepr. Rev. 57 Suppl. 3(1986)223-234.
3. GUELPA-LAURAS, C.-C, CARTEL, J.-L., CONSTANT-DESPORTES, M. MILLAN, J., BOBIN, P., GUIDI, C, BRUCKER, G., FLAGEUL, B., GUILLAUME, J.-C, PICHET, C, REMY, J.-C. and GROS SET, J. H. Primary and secondary dapsone resistance of M. leprae in Martinque, Guadeloupe, New Caledonia, Tahiti, Senegal, and Paris Between 1980 and 1985. Int. J. Lepr. 55(1987)672-679.
4. GUELPA-LAURAS, C.-C, GROSSET, J. H., CONSTANT-DÉPORTÉS, M. and BRUCKER, G. Nine cases of rifampin-resistant leprosy. Int. J. Lepr. 52(1984)101-102.
5. Jacobson, R. R. and Hastings, R. C. Rifampin-resistant leprosy. (Letter) Lancet 2(1976)1304-1305.
6. Ji, B. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
7. Languillon, J. Traitement de la maladie de Hansen par une dose unique de 1, 5 g de rifampicine associée à une sulfonothérapie continue. Med. Trop. 37(1977)717-719.
8. Languillon, J., Yawalkar, S. J. and Mc-Dougall, A. C. Therapeutic effect of adding rimactane 450 mg daily or 1200 mg once monthly in a single dose to dapsone 50 mg daily in patients with lepromatous leprosy. Int. J. Lepr. 47(1979)37-43.
9. Levy, L. Application of the mouse foot-pad technique in immunologically normal mice in support of clinical drug trials in lepromatous leprosy. Int. J. Lepr. 55Suppl.(1987)823-829.
10. Nollet, E., Janssens, L., Groenen, G., Bourland, J., Pattyn, S. and the Collaborative Study Group on the Treatment of Leprosy. Incubation time for relapse in multibacillary leprosy. Abstract in Int. J. Lepr. 52Suppl.(1984)686.
11. Pattyn, S. R. Incubation time of relapses after treatment of paucibacillary leprosy. Lepr. Rev. 55(1984)115-120.
12. Pattyn, S. R. Efficacy of different regimens in multibacillary leprosy. Lepr. Rev. 57 Suppl. 3(1986)265-271.
13. Pattyn, S. R. and Saerens, E. J. Minimal inhibitory dosage of rifampicin in intermittent treatment of Mycobacterium leprae infection in mice. Zentralbl. Bakteriol [A] 231(1975)503-507.
14. Shepard, C. C. The experimental disease that follows the injection of human leprosy bacilli into foot-pads of mice. J. Exp. Med. 112(1960)445-454.
15. Shepard, C. C. Recent developments in the chemotherapy and chemoprophylaxis of leprosy. Leprologia (Argen.) 19(1974)230-236.
16. Shepard, C. C. and McRae, D. H. A method for counting acid-fast bacteria. Int. J. Lepr. 36(1968)78-82.
17. Subcommittee on Clinical Trials of the Chemotherapy of Leprosy (THELEP) Scientific Working Group of the UNDP/World Bank/ WHO Special Programme for Research and Training in Tropical Diseases. Persisting Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58(1987)325-337.
18. Velleman, P. F. and Hoaglin, D. C, eds. Applications, Basics, and Computing of Exploratory Data Analysis. Boston: Duxbury Press, 1981, pp. 65-73.
19. Wehrli, W. Rifampin: mechanisms of action and resistance. Rev. Infect. Dis. 5 Suppl.3(1983)S407-S411.
20. Who Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.D., Professor; Faculté de Médecine Pitié-Salpêtrière, 91 Blvd. de l'Hôpital, 75634 Paris Cedex 13, France.
2. M.D., Assistant, Departement de Bactériologie-Virologie, Faculté de Médecine Pitié-Salpêtrière, 91 Blvd. de l'Hôpital, 75634 Paris Cedex 13, France.
3. M. D., Centre Hospitalier Territorial, Nouméa, New Caledonia.
4. M.D., Département de Parasitologic, Hôpital Pitié-Salpêtrière, Paris, France.
5. M.D., Institut Louis Malardé, Papeete, Tahiti.
6. M.D., Head, Departement de Dermatologie, Hôpital Clarac, Fort-de-France, Martinique.
7. M.D., Département de Dermatologie, Hôpital Saint-Louis, Paris, France.
8. M.D., Institut Pasteur, Guadeloupe.
9. M.D., Departement de Dermatologie, Hôpital Henri Mondor, Crétcil, France.
10. M.D., Director, Institut de Léprologie Appliquée, Dakar, Sénégal.
Received for publication on 22 December 1988.
Accepted for publication in revised form on 24 April 1989.