- Volume 57 , Number 3
- Page: 628–32
Susceptibilities of Mycobacterium leprae and M. avium complex to the H2O2-Fe-mediated halogenation system supplemented with antimicrobial agents
ABSTRACT
The susceptibilities of Mycobacterium leprae and M. avium complex (MAC) to the H2-O2-Fe-mediated halogenation system supplemented with antimicrobial agents were evaluated by fluorescein diacetateethidium bromide (FDA/EB) staining. In the case of M. leprae, the number of green-stained bacteria (intact cells) was reduced in the presence of the H2O2-Fe-mediated halogenation system supplemented with agents possessing antileprosy activity, such as rifampin, 4,4'-diaminodiphenylsulfone (dapsone), clofazimine, and ofloxacin. In the case of the MAC strain, although viable units of the organisms were reduced by the halogenation system alone, the number of green-stained cells in the FDA/EB stain was not reduced, even when the halogenation system was used in combination with ofloxacin. Because stainability of the cells is related to structural and functional intactness of the membrane, differences between M. leprae and the MAC strain imply possible differences in the rigidity of the cell membrane.RÉSUMÉ
On a évalué par une méthode de coloration par le diacétate-éthidium bromure de fluoresceine (FDA-EB), les susceptibilités respectives de Mycobacterium leprae et du complexe M. avium (MAC), au systeme d'halogénation par peroxyde d'oxygenc et fer interposes, auquel on avait ajouté des agents microbicides. Dans le cas de M. leprae, le nombre de bactéries colorées en vert (c'est-à-dire de cellules intactes), était réduit en présence du système d'halogénation décrit cidessus, lorsqu'on y avait ajouté des agents ayant une activité anti-lépreuse, tels que la rifampine, la 4,4',-diaminodiphényl-sulfone (dapsone), la clofazimine, et l'offoxacine. Dans le cas du complexe de MAC, malgré le fait que le nombre d'unités viables de ces organismes était réduit par le système d'halogénation utilisé seul, le nombre de cellules colorées en vert par le colorant FDA/EB n'était pas diminué, et ce en dépit du fait que le système d'halogénation était utilisé en combinaison avec l'ollaxacine. La colorabilité des cellules étant inllurcncée par l'intégrité structurelle et fonctionnelle de la membrane, les différences notées entre M. leprae et le complexe de M. avium dans cette étude suggèrent que la rigidité des membranes cellulaires de ces organismes pourrait présenter des differences.RESUMEN
Utilizando la tinción con diacetato de fluoresccinabromuro de etidio (DAF/BE) se evaluó la susceptibilidad del Mycobacterium leprae y del complejo del M. avium (MAC) a la halogenación mediada por el sistema H2O2-Fe suplementado con agentes antimicrobianos. En el caso dei M. leprae, el número de bactérias tenidas de verde (células intactas) se rejudo en presencia dei sistema de halogenación mediado por el H2O2-Fe suplementado con agentes antileprosos tales como rifampina, 4, 4'-diaminodifensilsulfona (dapsona), clofazimina y ofloxacina. En el caso del complejo MAC, aunque las unidades viables de los organismos disminuyeron en presencia solo del sistema de halogenación, el número de células tenidas de verde (tinción DAF/BE) no sc redujo ni siquiera cuando el sistema de halogenación se usó en combinación con la ofloxacina. Debido a que las características tintorialcs de las células están relacionadas con la integridad cstructural y funcional de la membrana, las diferencias entre M. leprae y MAC, implican posibles diferencias en la rigidez de la membrana celular.The H2O2-Fe-mediated halogenation system has a potent antimicrobial activity (4,9,10) a g a j ns i intracellular parasites, including mycobacteria (13), and it is thought to play an important role in the expression of antimicrobial activity of phagocytes. Mycobacterium leprae, an intracellular parasite, resists the bactericidal mechanisms of phagocytes. It is impossible to estimate the number of live organisms of M. leprae by colony counting, because M. leprae cannot be grown in vitro on any known media. It has been reported that fluorescein diacctatcethidium bromide (FDA/EB) staining is useful for determining the viability of mycobacterial cells, including M. leprae (12). This method is based on the following principle: FDA, a nonpolar and nonfluorescent fatty-acid ester, is transferred into intact cells through the cell membrane by an active transport system and is then hydrolyzed by cytoplasmic esterases, yielding polar fluorescent fluorescein which emits green light in response to excitation by ultraviolet light. Thus, viable cells are stained green. Ethidium bromide, on the other hand, can easily penetrate into dead cells through the damaged cell membrane but it is excluded by viable cells. EB is tightly bound to double-stranded DNA. This results in an orange fluorescence of dead cells (2,11).
We examined the susceptibility of M. leprae and a member of the M. avium complex (MAC) to the microbicidal activity of the halogenation system, with or without antimocrobial agents, using FDA/EB staining.
MATERIALS AND METHODS
Organisms. M. leprae Thai 53 was harvested from the foot pad tissue of nude mice infected with M. leprae obtained from K. Kohsaka, Research Institute for Microbial Diseases, Osaka University, Osaka, Japan. The M. leprae-infected foot pad tissue was minced with scissors and homogenized with a glass homogenizer in Hanks' balanced salt solution (HBSS) with 5% fetal bovine serum (FBS). This homogenate was centrifuged at 100 X g X 5 min and four fifths of the supernate was again centrifuged at 400 x g x 20 min. The pellet was suspended in HBSS with 5% FBS and centrifuged at 100 x g x 5 min. Three fifths of the supernate was diluted with 0.15 M saline to the appropriate concentration. The strain of the M. avium complex, ATCC 13950, supplied by A. Y. Tsang (National Jewish Hospital and Research Center, Denver, Colorado, U.S.A.) was cultivated in Dubos' Tween®-albumin medium (Eiken Chemical Co., Tokyo, Japan) at 37ºC for 7 days. The organisms were harvested by centrifugation (500 x g x 15 min), washed twice with 0.15 M saline, and resuspended in saline.
Chemicals. Fluorescein diacetate (FDA) and cthidium bromide (EB) were purchased from Sigma Chemical Co., St. Louis, Missouri, U.S.A. The antimicrobial agents used were: isonicotinic acid hydrazide (isoniazid, INH) (Wako Pure Chemical Co., Osaka, Japan); p-amino-salicylic acid (PAS) (Wako); 4,4'-diaminodiphenylsulfone (dapsone, DDS) (Nakarai Chemical Co., Kyoto, Japan); rifampin (RFP) (Daiichi Pharmaceutical Co., Tokyo, Japan); ofloxacin (OFLX) (Daiichi); cefoxitin (CFX) (Daiichi); clofazimine (CLO) (CIBA-GEIGY Co., Hyogo, Japan); and cofetatan (CTT) (Yamanouchi Pharmaceutical Co., Tokyo, Japan).
Antimycobacterial activity of the halogenation system. The microbicidal activity of the halogenation system was measured as described previously (13). Briefly, the reaction mixture (2 ml), consisting of 100 μM sodium iodide, 100 μM hydrogen peroxide, 100 μM ferrous sulfate, various concentrations of antimicrobial agents, 20 mM acetate buffer (pH 5.5) and test organisms (at a final concentration of 1 x 107) organisms/ml), was incubated in a shaking water bath at 37ºC for 60 min. The viability of the M. leprae in the resulting incubation mixture was determined by FDA/EB staining. In the case of the M. avium complex strain, the number of residual colony-forming units (CFU) in the incubation mixture was estimated by plating on Middlebrook 7H10 agar plates (Difco Laboratories, Detroit, Michigan, U.S.A.).
FDA/EB staining. FDA/EB staining was done by the method of Tsukiyama (12). Briefly, the reaction mixture was diluted with 4 ml of phosphate buffered saline (PBS) (pH 7.2) after incubation and centrifuged at 1000 x g x 30 min. The organisms were suspended in 0.1 ml of Dubos' Tween®-albumin medium and smeared on a glass slide. The FDA/EB solution consisted of 0.5 ml of 1 mg/ml FDA in acetone, 0.02 ml of 2 mg/ml EB in PBS (pH 6.5) containing 0.05% Tween® 80 (PBS-Tween® 80) and 4.5 ml of PBS-Tween® 80. After the smear had been incubated in the FDA/EB solution at 37ºC for 60 min, the numbers of green- and orange-stained cells were counted at a magnification of 400 under a fluorescent microscope. The percentage of green-stained cells in the total cells (green- plus orange-stained cells) was then calculated.
RESULTS
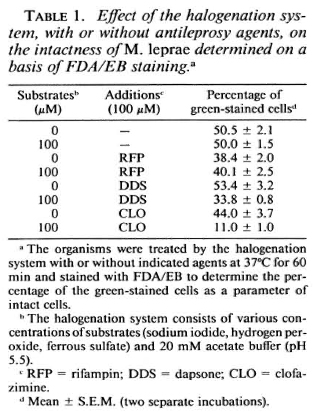
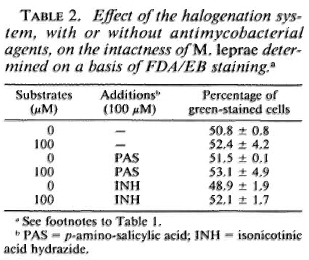
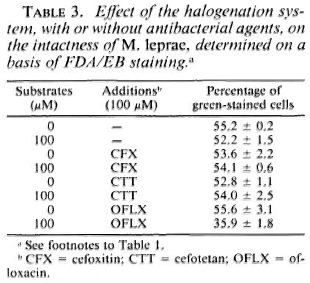
Susceptibility of M. leprae to the halogenation system with or without antimicrobial agents. Table 1 shows the changes in the viability of M. leprae exposed to the halogenation system, with or without the addition of antileprosy agents, as determined by FDA/EB staining. The percentage of green-stained cells was not affected by the halogenation system alone but was reduced by the system combined with either RFP, DDS, or CLO. It is noted that RFP alone reduced the viability of the organisms. The synergistic effect was highest with CLO, followed by DDS. In cases of the antituberculosis drugs (Table 2) such as PAS and INH, there was no such decrease. As shown in Table 3, neither CFX nor CTT caused a significant decrease in green-stained cells, even in combination with the halogenation system; the halogenation system plus OFLX caused a marked reduction in the viability of the organisms.
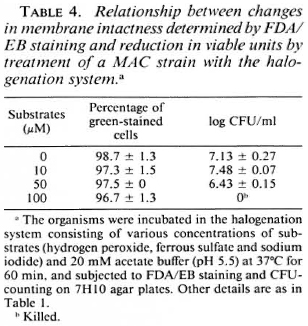
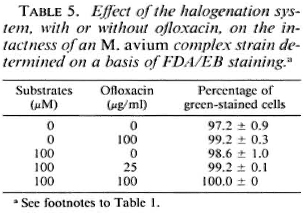
Susceptibility of M. avium complex to the halogenation system. Table 4 shows the susceptibility of the MAC strain to the halogenation system on the basis of changes in viable units and in the ratio of green-stained cells after FDA/EB staining. The halogenation system did not afTect the behavior of the organisms in FDA/EB staining, but markedly reduced the number of residual CFU. This indicates that, in the case of the MAC strain, the ability of the organisms to be stained green by FDA/EB did not correlate with the ability to grow on 7H10 agar medium. Table 5 shows the effects of the halogenation system supplemented with OFLX on the grccn-fluorcscence intensity of the MAC strain. Even in the presence of OFLX, the halogenation system failed to reduce the number of the green-stained organisms by FDA/EB staining.
DISCUSSION
The peroxidase- or Fe-mediated halogenation system has potent bactericidal activity (4.9,10.13) This antimicrobial mechanism seems to be mediated by iodination and oxidation of cellular components requisite for viability, such as membrane-proteins and iron sulfur centers of the electron transport system (4,9,10). FDA is taken up by cells due to an active transport system of the cell membrane and subsequently is hydrolyzcd by intracellular esterases, giving a green fluorescence. EB enters cells through the damaged cell membrane and produces an orange fluorescence by binding to DNA. These events lead to a reduction of green fluorescence and an increase in orange photocmission in the case of a damaged cell membrane (12). Here, we found that the H2O2-Fe-mediated halogenation system alone did not reduce the green-fluorescence intensity of M. leprae or the MAC strain. Therefore, it seems that the halogenation system alone failed to cause damage to the cell membrane of M. leprae, at least not to a degree that resulted in the reduction of its green fluorescence.
On the other hand, when the halogenation system was supplemented with OFLX, so-treated M. leprae showed a decrease in the number of green-stained cells in FDA/EB staining. OFLX primarily inhibits the supercoiling activity of DNA gyrasc, and thus it does not directly cause any cell membrane damage (3). Therefore, it is thought that the halogenation system, even combined with OFLX, could not damage the cell membrane structure. However, it is still possible that the antimicrobial system might inactivate the active transport system of the cell membrane in an indirect manner, causing reduction of the green fluorescence of treated organisms under FDA/EB staining.
DDS and CLO, which do not have a primary action of the cell membrane (1,7,8), also caused a decrease in the number of green-stained M. leprae when used in combination with the halogenation system. The mechanisms of the cell membrane function-disintegration action of these agents may be similar to that of OFLX. RFP alone reduced the percentage of green-stained cells. However, RFP is known to primarily inhibit the RNA polymerase of organisms (6). In studies using an electron microscope, damage to the structure of the cytoplasm, ribosomes and mesosome of the organisms by RFP was observed, but structures of the nucleus, cytoplasmic membrane and cell wall were preserved (5). Thus, while RFP does not cause structural damage to the cell membrane, it is possible that the integrity of the cell membrane of the organism might be reduced when protein synthesis is inhibited by RFP.
In the case of the MAC strain, the number of viable units was decreased by the halogenation system but the green-fluroescence intensity of the organisms in FDA/EB staining was not affected, even when the halogenation system was combined with OFLX. This implies that the halogenation system may inactive certain cell components essential for growth of the organisms without damaging the active transport activity or the integrity of the cell membrane. These results suggest that the functional state of the M. leprae cell membrane is much more susceptible to damaging action of the halogenation system supplemented with OFLX than is the MAC strain cell membrane. If such is the case, the structural and functional stability of the M. leprae cell membrane, including the active transport system, is thought to be much less than that of the MAC strain cell membrane.
In any case, this study revealed that a reduction of the green fluorescence of M. leprae cells in FDA/EB staining was produced when M. leprae cells were treated with the Fe-mediated halogenation system combined with an anti-M leprae agent. This sytem may be useful for in vitro screening of antileprosy agents.
REFERENCES
1. BROWN, G.M. Inhibition by sulfonamides of the biosynthesis of folic acid. Int. J. Lepr. 35(1967)580-589.
2. HOLLANDER, D. H., LITTON, L. E. and LIANG, Y. W. Ethidium bromide countcrstain for differentiation ofquinacrinc stained interphase bodiesand brilliant mctaphasc bands. Exp. Cell Res. 99(1976)174-175.
3. IMAMURA, M., SHIISAMURA, S., HAYAKAWA, I. and OSADA, Y. Inhibition of DNA gyrasc by optically active ofloxacin. Antimicrob. Agents Chemother. 31(1987)325-327.
4. KLEBANOFF, S. J. Iodination of bacteria: a bactericidal mechanism. J. Exp. Med. 126(1967)1063-1079.
5. KONNO, K., OIZUME, K., ARIJI. F., YAMAGUCHI, J. and OKA, S. Mode of action of rifampin on mycobacteria. I. Electron microscopic study of the effect of rifampin on Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 107(1973)1002-1005.
6. KONNO, K., OIZUME, K. and OKA, S. Mode of action of rifampin on mycobacteria. II. liiosynthetic studies on the inhibition of ribonucleic acid polymerase of Mycobacterium bovis BCG by rifampin and uptake of rifampin--14C by Mycobacterium phlei. Am. Rev. Respir. Dis. 107(1973)1006-1012.
7. LEVY, L. Pharmacologic studies of clofazimine. Am. J. Trop. Med. Hyg. 23(1974)1097-1109.
8. NIWA, Y. and OZAKI, M. Possible action mechanism of antilcprotic agents clofazimine in the phagocytes of leprotic patients in association with pathogenesis of Hansen's disease. Jpn. J. Clin. Hematol. 24(1983)1039-1048.
9. ROSEN, H. and KLEBANOFF, S. J. Oxidation of Escherichia coli iron centers by the myeloperoxidasc-mcdiated microbicidal system. J. Biol. Chem. 257(1982)13731-13735.
10. ROSEN, H. and KLEBANOFF, S. J. Oxidation of microbial iron-sulfur centers by the myeloperoxidase-H2-O2-halide antimicrobial system. Infect. Immun. 47(1985)613-618.
11. ROTMAN, B. and PAPERMASTER. B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc. Natl. Acad. Sci. U.S.A. 55(1965)134-141.
12. TSUKIYAMA, F., KATOH, M. and MATSUO, Y. Modification of the fluorescent staining method for mycobacterial cells. Hiroshima J. Med. Sci. 33(1984)293-295.
13. YAMADA, Y., SAITO, H., TOMIOKA, H. and JIDOI, J. Relationship between the susceptibility of various bacteria to active oxygen species and to intracellular kiling by macrophages. J. Gen. Microbiol. 133(1987)2015-2021.
1. Ph.D.; Department of Microbiology and Immunology; Shimane Medical University, Izumo 693, Japan.
2. M.D., Ph.D., Department of Microbiology and Immunology; Shimane Medical University, Izumo 693, Japan.
3. M.D., Ph.D.; Department of Dermatology, Shimane Medical University, Izumo 693, Japan.
4. M.D., Ph.D., Department of Dermatology, Shimanc Medical University, Izumo 693, Japan.
Received for publication on 21 March 1989.
Accepted for publication on 24 April 1989.