- Volume 57 , Number 3
- Page: 633–40
Lymphoproliferation and in vitro antibody synthesis in leprosy patients
ABSTRACT
An in vitro system to assess B-cell function in leprosy patients is described. In vitro lymphoprolifcration and antibody synthesis by peripheral blood mononuclear cells (PBMC) in response to pokewced mitogen (PWM) and Formalin-treated Staphylococcus aureus Cowan I (FSA) f rom 31 leprosy patients and 13 healthy controls were studied. DNA synthesis was induced by both PWM and FSA in PBMC f rom all of the leprosy patients and control subjects. Lepromatous leprosy (LL) patients' cells showed higher responses to both PWM and FSA. However, these increases were not statistically significant. The levels of secreted IgM, IgG, or IgA were examined in the 7-day culture supernatants of PBMC cultured with or without PWM or FSA using an enzyme-linked immunosorbent assay. Wide individual variations were observed in in vitro antibody synthesis. IgM secretion in PBMC f rom normal subjects and various groups of leprosy patients in response to PWM and FSA was comparable. In vitro IgG secretion in response to PWM was the highest in cells f rom LL patients; it was significantly decreased in cells f rom tuberculoid leprosy (TT) patients (p < 0.01). The levels in cells f rom borderline leprosy (BB) patients were intermediate in response to the same mitogen. Cells f rom leprosy patients as a group showed a higher spontaneous secretion of IgA in comparison with cells f rom normal subjects. Overall, the in vitro Ig secretion by PBMC in different patient groups appears to reproduce the spectrum of antibody levels observed in patients in vivo. Thus, the present in vitro culture system may help to de linéate the mechanisms of B-cell dysregulation in leprosy.RÉSUMÉ
On décritici un système in vitro mis au point en vue d'évaluer la fonction des cellules B chez des malades de la lèpre. Chez 31 malades de la lèpre et chez 13 témoins en bonne santé, on a étudié la lymphoproliferation in vitro, de même que la synthèse par la cellules mononucléaires du sang périphérique (PBMC) en réponse à un mitogène de phytolacca (poke weed) (PWM) et par l'antigène Cowan 1 de Staphylococcus aureus (FSA) traité par la formaline. Une synthèse d'ADN a été induite tant par PWM que par FSA dans des cellules mononucléaires du sang périphérique, dans tous les échantillons qu'ils proviennent de malades de la lèpre ou de sujets témoins. Les cellules recueillies chez les malades atteints de lèpre lépromatcusc (LL) ont témoigné d'une réponse plus forte tant au PWM qu'au FSA. Néanmoins, les augmentations qui ont été notées n'étaient pas statistiquement significatives. On a utilisé un ELISA pour étudier les taux d'IgM, d'IgG, ou d'IgA sécrétés, dans des supernageants de cultures âgées de 7 jours de cellules mononucléaires cultivées avec PWM ou FSA. On a relevé de larges différences dans les réactions individuelles de la synthèse in vitro d'anticorps. La sécrétion d'IgM en réponse au PWM et au FSA par les cellules mononucléaires provenant de sujets normaux, ainsi que par celles provenant de différents groupes de malades de la lèpre, étaient comparables. La sécrétion in vitro d'IgG en réponse au PWM était la plus élevée dans les cellules provenant de malades LL. Elle était significativement diminué dans les cellules obtenues chez des malades atteints de lèpre tubcrculoïde (TT) (p < 0,01). Les taux relevés dans les cellules de malades atteints de lèpre dimorphe (BB) étaient intermédiaires en ce qui concerne les réponses à l'un ou autre de ces mitogènes. Les cellules de malades de la lèpre, pris en tant que groupe sans égard à leur type, présentaient une sécrétion spontanée plus élevée d'IgA, que les cellules provenant de sujets normaux. De manière globale, la sécrétion in vitro d'Ig par les cellules mononucléaires provenant du sang périphérique, dans différents groupes de malades, semble reproduire le spectre des taux d'anticorps observés in vivo chez des malades. Dès lors, on en conclut que le système de culture décrit ici pourrait être utile pour définir les mécanismes de la dérégulation des cellules B dans la lèpre.RESUMEN
Se describe un sistema in vitro para evaluar la función de las células B en los pacientes con lepra. Se estuidiaron la linfoproliferación in vitro y la síntesis de anticuerpo por las células mononucleares de sangre periférica (CMSP) en respuesta al estímulo con fitolaca americana (pokewecd mitogen, PWM) y al Staphylocoecus áureas (Cowan I) tratado con formalina (SAF). El estudio se hizo en 31 pacientes con lepra y en 13 individuos control. La síntesis de DNA se indujo tanto por el PWM como por el SAF en las CMSP de todos los pacientes con lepra y en las de los controles sanos. Las células de los pacientes con lepra lepromatosa (LL) mostraron las respuestas más elevadas tanto al PWM como al SFA. Sin embargo, estos incrementos no fueron estadísticamente signifactivos. Usando un inmunoensayo enzimático, se midió la secreción de IgM, IgG y de IgA, en los sobrenadantes de CMSP cultivadas por 7 días con o sin PWM o FSA. Se observaron amplias variaciones individuales en la síntesis in vitro de anticuerpos. La secreción de IgM en respuesta a la estimulación con PWM y FSA por las CMSP de los sujetos sanos fue comparable a la observada en los pacientes con los diversos tipos de lepra. La secreción in vitro de IgG en respuesta al PWM fue mas elevada en los pacientes con LL y estuvo significativamente disminuida en los pacientes con lepra tubcreuloide (TT) (p < 0.01). En las células de los pacientes con lepra intermedia (BB) la respuesta al mismo mitógeno fue también intermedia. Las células de los pacientes con lepra mostraron mayor secreción espontánea de IgA que las células de los individuos control. De manera general, la secreción in vitro de Igs por las CMSP de los diferentes grupos de pacientes, parece reproducir el espectro de los niveles de anticuerpos observado in vivo en los pacientes. Así, el presente sistema de cultivo in vitro puede ayudar a delinear los mecanismos de desregulación de las células B en los pacientes con lepra.Human leprosy, caused by a noncultivable mycobacterium, Mycobacterium leprae, represents a model immunological disorder. The immune responses of leprosy patients during the course of the natural infection have been extensively studied over the last decade (10,16). It is now well established that protection against the disease in healthy subjects and the localized nature of tuberculoid leprosy (TT) correlates directly with specific cell-mediated immune response (CMIR). On the other hand, specific ancrgy of CMIR to M. leprae antigens is seen in lepromatous leprosy (LL) patients, and this is accompanied by an enhanced humoral response to M. leprae-specific antigens, to M. leprae anitgens crossreacting with other mycobacteria, and to self components (9). Humoral responses in tuberculoid leprosy are low. The mechanism underlying this inverse relationship between cellular and humoral responses evoked by M. leprae is still incompletely understood. Recent evidence from our laboratory indicates that suppressor-T cells may play a role in switching off antibody responses in tuberculoid leprosy and the paucity of such regulatory T cells in LL patients may underlie the enhanced humoral immune responses (18,19). Clearly, direct studies aimed at understanding the basis of these dysrcgulatcd B-cell responses are required.
The present study was undertaken to evaluate the function of B cells in patients across the leprosy spectrum. Both in vitro antibody synthesis and DNA synthesis by B cells were examined using pokewecd mitogen (PWM), a T-cell dependent B-cell activator (6), and Formalin-treated Staphylococcus aureus Cowan I (FSA), which is reported to stimulate B cells in a suppressor-T-cell independent manner (20).
MATERIALS AND METHODS
Subjects
Controls. Thirteen healthy volunteers, matched for age and sex and ethnic origin with the patients, were included as controls.
Patients. Eighteen lepromatous (LL), 8 borderline (BB) and 17 tuberculoid (TT) leprosy patients attending the outpatient clinics of the departments of dermatology at the All India Institute of Medical Sciences and the Safdarjang Hospital, New Delhi, India, were included in the study. The patients were classified according to the Ridley and Jopling classification (22) based on clinical and histological features.
Culture conditions and assays
Culture medium. Complete culture medium consisted of RPMI 1640 (GIBCO, Grand Island, New York, U.S.A.) supplemented with 20 raM HEPES, 2 g/1 sodium bicarbonate, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 raM L-glutamine, 5 x 10-5 M 2-mercaptoethanol.
Separation of peripheral blood mononuclear cells. Peripheral blood mononuclear cells (PMBC) were isolated from heparinized venous peripheral blood by Ficoll-Paque (Pharmacia Fine Chemicals, Uppsala, Sweden) gradient centrifugation (3). PBMC were washed five times with Hank's balanced salt solution (HI3SS) (GI13CO) containing 5% fetal calf serum (FCS). The cells were resuspended in complete culture medium and viability was checked by the trypan blue dye exclusion method. Viability was routinely > 95%.
The percent of sheep erythrocyte rosette forming cells (mean ± S.D.: LL = 59 ± 5.0, BB = 58 ± 3.9, TT = 61 ± 4.2 and controls = 62 ± 3.6); surface immunoglobulin positive cells (mean ± S.D.: LL = 16 ± 2.5, BB = 15.2 ± 3.1, TT = 15.5 ± 3.6 and controls = 14.5 ± 4.2) and nonspecific esterase-positive cells (mean ± S.D.: LL = 18.6 ± 2.2, BB = 16.5 ± 1.7, TT = 17.2 ± 2.0 and controls = 18.1 ± 1.5) among PBMC were enumerated.
Preparation of FSA. FSA was prepared by the method of Kessler (12). Briefly, isolated colonies of S. aureus Cowan I strain obtained from the Department of Microbiology, All India Institute of Medical Sciences, New Delhi, were selected from sheep blood agar plates. Mass culture was prepared by overnight incubation at 37ºC of twice passaged bacteria in trypticasc soy broth (BBL, Microbiology Systems, Cockeysville, Maryland, U.S.A.). The bacteria were harvested and washed in phosphate buffered saline (PBS). A bacterial pellet from a one-liter culture was resuspended in 30 ml PBS, and the bacteria were treated with 1.5% Formalin for 90 min at room temperature. After washing with PBS, the bacteria were killed at 80ºC for 5 min and rapidly cooled in an icewatcr bath for 5 min. After two more washes in sterile PBS the concentration was adjusted to 10% (v/v). These Formalin-treated and heat-killed bacteria were stored at 4ºC.
Lymphoproliferation. Four replicates of 10s PBMC in 100 μl of complete culture medium with 10% human AB serum were added per well in 96-well, round-bottom tissue culture plates (NUNC, Intermed, Denmark). Twenty-five μl of the optimal concentration of PWM (1:250 v/v), FSA (0.00625% v/v), or medium was added and the cells were cultured for 6 days at 37ºC in a humidifed atmosphere of 5% CO2 and 95% air. Eighteen hr prior to termination of the culture, 0.5 μCi of 3H-methylthymidine was added to each well (Radiochemical Centre, Amersham Corporation, Arlington Heights, Illinois, U.S.A.; specific activity 2 Ci/ mMole). Harvesting was done on a semiautomatic harvester (PHD cell harvester; Cambridge Technology Inc., Cambridge, Maryland, U.S.A.) using glass-fiber filters (GF/C; Whatman, England). Radioactivity was counted in a liquid scintillation counter (Rack beta; LK.B, Finland) using a toluene-based scintillation cocktail.
In vitro antibody synthesis. The cultures were set up in quadruplicate in 96-well, fiat-bottom tissue culture plates (NUNC); 2 x 105 cells in 200 μl culture medium with 10% FCS were seeded per well. Then 50 μl of medium, PWM (1:50 v/v), or FSA (0.00625% v/v) was added. The concentrations of mitogens were determined to be optimal in preliminary experiments. The cells were incubated at 37ºC in a humidifed atmosphere of 5% CO2 and 95% air for 7 days. The plates were then centrifuged at 400 x g x 10 min. The supernatants from replicates were pooled and stored at -20ºC until assayed for immunoglobulins by enzyme-linked immunosorbant assay (ELISA).
Estimation of class-specific immunoglobulins by ELISA. Total immunoglobulins (IgM/IgG/IgA) present in the culture supernatants were estimated by sandwich ELISA method (24). Polystyrene plates (NUNC Immunoplatc I) were coated by adding 200 μl/well of 5 μg of rabbit antihuman immunoglobulin heavy chain (γ/μ/α) antibody (Dakopatts, Denmark) in 0.1 M sodium carbonate buffer, pH 9.6, overnight at 4ºC. The wells were washed three times in PBS containing 0.05% Tween 20 (Sigma Chemical Co., St. Louis, Missouri, U.S.A.). The unbound sites were blocked by incubation with 2% BSA in PBS for 1-2 hr at room temperature. The plates were washed and 200 μl of sample supernatants or known standards of human immunoglobulin heavy chains (γ/μ/α) were added to each well. For each test, three appropriate dilutions of the culture supernatant and eight concentrations ranging from 10 to 100 ng/ml of chromatographically purified heavy chains were used. Dilutions were made in 1% BSA in PBS/Tween. After incubation at room temperature for 3 hr, the plates were washed and incubated with corresponding anti-human Ig heavy-chain-specific antibody conjugated to horseradish peroxidase for 3 hr.
After the plates were washed, 200 μl of the substrate was added per well and incubated for 30 min at 37ºC. The substrate solution consisted of 1 mg/ml of ortho-phenylene diamine (OPD) (Sigma) in citrate phosphate buffer (0.1 M, pH 5.0). The reaction was stopped by adding 25 μl of 4 N sulfuric acid per well. The optical density (OD) was measured at 492 nm in a semiautomatic ELISA reader (Dynatech, Springfield, Virginia, U.S.A.). All of the assays were performed in duplicate, and appropriate controls and blanks were included in each assay.
Standard curves were constructed by plotting the OD against the concentrations of standards. Concentrations with absorbance values within the linear part of the curve were used as references. The relationship of absorbance and concentration was expressed by the formula Y = aX + b, where a and b were the constants. For calibrations, concentration was expressed as function (Y) of absorbance (X). The constants were calculated by solving the following equations:
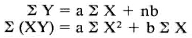
Statistical analysis. Values of the groups were expressed as arithmetic or geometric means (GM) with standard deviations. For comparison, original values were converted into logarithmic values if the original values did not follow a normal distribution. Comparisons were made using one-way analysis of variance followed by multiple range.
RESULTS
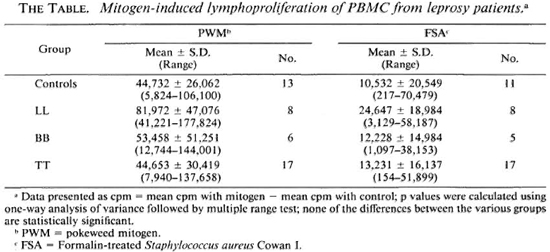
Lymphoproliferation. The proliferative responses of PBMC from leprosy patients and controls are presented in The Table. The responses of tuberculoid leprosy patients and control subjects to PWM are comparable. PBMC from lepromatous leprosy patients showed higher proliferative responses to PWM when compared with the control group. The response of borderline individuals was intermediate between that of LL and TT. Proliferative responses to FSA showed a pattern similar to that observed with PWM. The differences in lymphoproliferative responses of the different groups either to PWM or to FSA were not statistically significant.
Spontaneous in vitro immunoglobulin secretion by PBMC. IgG, IgM and IgA were estimated in 7-day culture supernatants of PBMC derived from 30 leprosy patients and 11 healthy volunteers. The results of these analyses are shown in Figure 1. Similar levels of IgM and IgG were observed in PBMC culture supernatants from healthy volunteers and TT patients. Patients with lepromatous and borderline leprosy showed enhanced levels of spontaneous immunoglobulin secretion in vitro. However, the differences are not statistically significant compared to controls. As expected, the levels of IgG were the highest. Interestingly, IgG levels were found to be higher than IgM levels in all subjects. The pattern of spontaneous IgM secretion was similar to that of IgG, with higher levels being observed in lepromatous and borderline leprosy patients. IgA levels in leprosy patients showed higher values than normal; however, these differences are statistically insignificant.
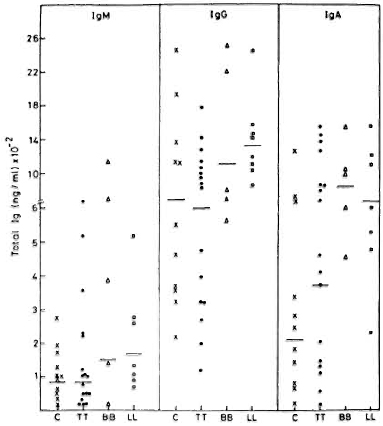
Fig. 1 Spontaneous secretion of IgM, IgG, and IgA by PBMC from control subjects (C), and tuberculoid (TT), borderline (BB), and lepromatous (LL) leprosy patients. Immunoglobulin levels were measured by ELISA. Individual values and geometric means (-) for each group are depicted.
Mitogen-induced in vitro immunoglobulin synthesis. The levels of IgG secreted by PBMC from normal subjects and leprosy patients are shown in Figure 2. Healthy volunteers showed higher IgG secretion when PBMC were stimulated by PWM (geometric mean = GM = 4915.5 x ÷ 1.7 ng/ml) compared to stimulation with FSA (GM = 1630.4 x ÷ 3.1 ng/ml). Leprosy patients also showed a similar pattern. Individual variations were observed with both mitogens in normal subjects as well as in leprosy patients. Among the leprosy patients, the lepromatous group showed the highest levels of IgG with both PWM (GM = 5020 x ÷ 2.9 ng/ml) and FSA (GM = 2466.3 x ÷ 2.0 ng/ml). * The mean IgG level in LL patients was similar to that of normal healthy controls. BB patients showed intermediate levels of IgG secretion when compared to LL patients and control subjects in response to PWM (GM = 2380.7 x ÷ 2.4 ng/ml) and FSA (GM = 1482.6 x ÷ 2.7 ng/ml). Tuberculoid leprosy patients, on the other hand, showed strikingly lower IgG production with PWM (GM = 1198.8 x ÷ 2.3 ng/ml) when compared to the LL and BB patients, and the control subjects (P < 0.01). IgG produced in response to FSA (GM = 777 x ÷ 4.2 ng/ml) by PBMC from TT patients is not statistically different compared to the other groups.
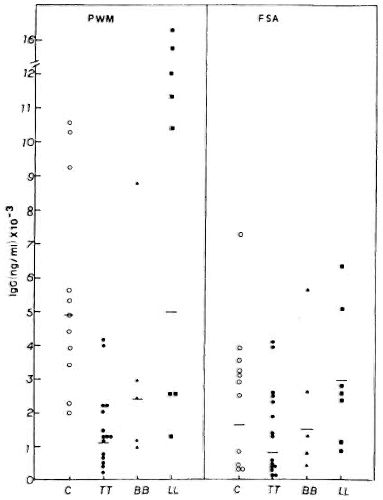
Fig. 2. PWM- or FSA-induced IgG secretion by PBMC from C, TT, BB, and LL patients, showing individual values and geometric means (-) for each group.
Like the IgG response, PWM stimulated IgM synthesis better when compared to FSA in both control subjects and leprosy patients (Fig. 3). Wide ranges in individual values were again observed in all groups studied. In general, the PWM-induced IgM production was similar in normal subjects (GM = 1916.2 x ÷ 2.8 ng/ml) and in lepromatous leprosy patients (GM = 1823.2 x ÷ 3.6 ng/ ml); whereas much lower levels were found in BB (GM = 571.6 x ÷ 8.0 ng/ml) and tuberculoid leprosy patients (GM = 671.0 x ÷ 5.4 ng/ml). There were no statistically significant differences between control subjects and leprosy patients or among leprosy patients. In contrast, FSA induced higher IgM levels in LL (GM = 1026.7 x ÷ 3.2 ng/ ml) and BB patients (GM = 1253.3 x ÷ 2.3 ng/ml) as compared to the control subjects (GM = 340.0 x ÷ 4.9 ng/ml) and TT patients (GM = 337.9 x ÷ 4.1 ng/ml).
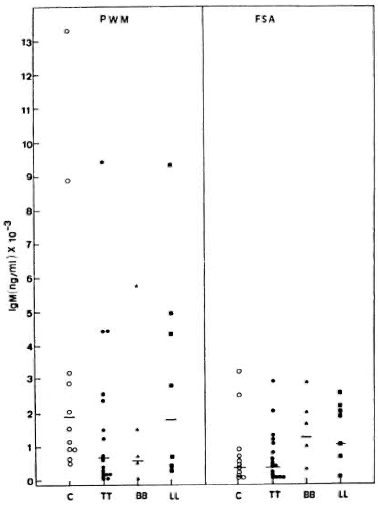
Fig. 3. PWM- or FSA-induced IgM secretion by PBMC from C, TT, BB, and LL patients, showing individual values and geometric means (-) for each group.
Interestingly, all groups of subjects showed higher IgA than IgM secretion, both spontaneously and after stimulation with PWM (Fig. 4). Again, PWM was a better inducer than FSA. In contrast to a differential production of IgG and IgM, similar IgA production was observed in response to each mitogen in the various groups of leprosy patients (LL, TT and BB). IgA levels in tuberculoid patients in response to PWM (GM = 441 x ÷ 3.6 ng/ml) and FSA (GM = 595 x ÷ 3.3 ng/ml) were similar to those of normal subjects (GM = 493 x ÷ 4.2 ng/ml with PWM and GM = 260 x ÷ 3.8 ng/ml with FSA). The LL (GM = 687.3 x ÷ 4.6 ng/ml with PWM and GM = 630.6 x ÷ 2.3 ng/ml with FSA) and BB patients (GM = 944.7 x ÷ 1.4 ng/ml with PWM and GM = 1395.2 x ÷ 1.7 ng/ml with FSA) showed higher levels.
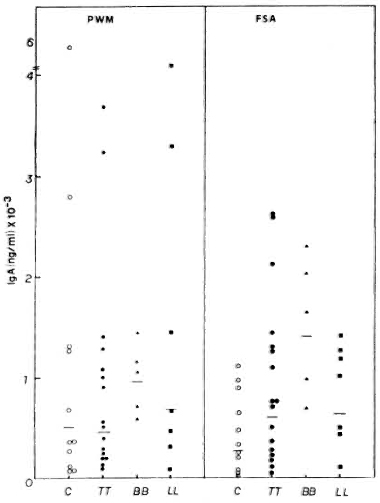
Fig. 4. PWM- or FSA-induced IgA secretion by PBMC from C, TT, BB, and LL patients, showing individual values and geometric means (-) for each group.
DISCUSSION
In the present study, in vitro responses of PBMC from leprosy patients to PWM and FSA were assessed by lymphoproliferation and immunoglobulin synthesis. Lymphoproliferation to PWM and FSA was higher in the lepromatous group as a whole compared to the tuberculoid patients and to the controls. However, this enhancement was not statistically significant. These observations are in contrast to earlier reports where generalized responses of PBMC were reported to be reduced in lepromatous patients (5-15). This discrepancy may be due to the different inducers; in the earlier studies T-cell mitogens were used while in the present study we used PWM, which induces T cells and also B cells when T cells are present (7,11) and FSA, which induces mainly the proliferation of B cells (8,23).
Immunoglobulin secretion was assessed by estimating both spontaneous Ig release and that following polyclonal stimulation. The borderline and lepromatous patients showed enhanced spontaneous secretion of Ig in vitro; IgG secretion was highest, followed by IgA and then IgM. Since the cultures were maintained in fetal calf serum after multiple washings of cells, it is very unlikely that the Ig in culture supernatants was due to carry-over effects. The present results are consistent with other reported studies. Bullock, et al. (4), using a reverse plaque assay, also investigated this problem and concluded that spontaneous PFCs of LL patients were higher compared to healthy controls. In their studies as well, a wide individual variation in the number of PFCs in each group was observed. Lai A Fat, et al. (13) showed spontaneous secretion of Ig and complement in lesional tissues of LL patients.
Another interesting feature of the present study was the enhanced IgA levels observed in the culture supernatants. Since Indian subjects are known to encounter numerous gastrointestinal pathogens, it is possible that the raised IgA levels may reflect a response to such infections. In this regard, it is pertinent that Abe, et al. (1) observed high IgA anti-M. leprae antibodies in LL patients using a fluorescent antibody technique.
The PBMC of all of our subjects responded to in vitro polyclonal stimulation with PWM and FSA. LL patients responded to a degree similar to control subjects. Again, IgA secretion was enhanced in LL patients. These findings are comparable with the data of Abe, et al. (2) as discussed earlier and may indicate a unique in vivo response to M. leprae.
The decrease in IgG secretion in tuberculoid leprosy individuals after PWM stimulation may reflect immunoregulatory events in this type of leprosy. Extensive data from our laboratory indicate increased suppressor-T cells in this group of patients which has been interpreted as a physiological regulatory control initiated by natural infection (17,18). Bullock, et al. (4) have shown aberrant immunoregulatory control of B-cell activation in vitro when co-cultured with T cells from normal subjects.
The present study thus demonstrates that leprosy patients display a spectrum of in vitro B-cell immune responsiveness which directly parallels their antibody status in vivo. It should now be possible to study the mechanisms of dysregulation of B-cell function and the role of T cells using this convenient in vitro system.
Rawlinson, et al. (21) reported an increase in autoantibodies in serum immunoglobulins in lepromatous patients. Similarly, it was shown that monoclonal antibodies derived from PBMC of lepromatous leprosy patients fused after in vitro stimulation with PWM showed reactivity to self-antigens and shared idiotypic specificity to monoclonal antibodies derived from systemic lupus erythematosus patients (14). It will be of great interest to study the reactivity of the antibodies to various mycobacterial and sclfantigens using the present system of in vitro B-cell activation.
Acknowledgments. We thank Dr. Hamid Band for his critical reading of the manuscript. Chandri Naidu Yandava was supported by a fellowship from the All India Institute of Medical Sciences, New Delhi, India.
REFERENCES
1. ABE, M., IZUMI, S., SAITO, T. and MATHUR, S.K., Early serodiagnosis of leprosy by indirect immunofluorescence. Lepr. India 48(1976)272-276.
2. ABE, M., YOSHINO, Y., MINAGAWA, F., MIYAJI, I., SAMPOONACHOT, P., OZAWA, T., SAKAMOTO, Y., SAITO, T. and SAIKAWA, K. Salivary immunoglobulins and antibody activities in leprosy. Int. J. Lepr. 52(1984)343-350.
3. BOYUM, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21 Suppl. 97(1968)77-79.
4. BULLOCK, W. E., WATSON, S., NELSON, K. E., SHAUF, V., MAKONKAWKEYOON, S. and JACOBSON, R. R. Aberrant immunoregulatory control of B lymphocyte function in lepromatous leprosy. Clin. Exp. Immunol. 49(1982)105-114.
5. DIERKS, R. E. and SHEPARD, C. C. Effect of phytohemagglutinin and various mycobacterial antigens on lymphocyte cultures from leprosy patients. Pro. Soc. Exp. Biol. Med. 127(1968)391-395.
6. FAUCI, A. S. Human B cell function in a polyclonally induced plaque forming cell system. Cell triggering and immunoregulation. Transplant. Rev. 45(1979)93-116.
7. FAUCI, A. S., PRATT, K. R. and WHALEN, G. Activation of human B lymphcytcs. II. Cellular interactions in the PFC response of human tonsillar and peripheral blood B lymphocytes to polyclonal activation by pokeweed mitogen. J. Immunol. 117(1976)2100-2104.
8. FORSGREN, A., SVEDJELUND, A. and WIGZELL, H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur. J. Immunol. 6(1976)207-213.
9. GODAL, T. Immunological aspects of leprosy-present status. Prog. Allergy 25(1978)211-242.
10. GODAL, T. Leprosy immunology -some aspects of the role of the immune system in the pathogenesis of disease. Lepr. Rev. 55(1984)407-414.
11. KEIGHTLEY, R. G., COOPER, M. D. and LAWTON, A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J. Immunol. 117(1976)1538-1544.
12. KESSLER, S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody absorbent: parameters of the interaction of antibody-antigen complexes with protein A. J. Immunol. 115(1975)1617-1624.
13. LAIA FAT, R. F., JIN, J. C, DIESSELHOFF-DEN DULK, M. and VA N FURTH, R. in vitro synthesis of humoral factors (immunoglobulins and complement) in lcsional skin of leprosy patients. Infect. Immun. 25(1979)891-895.
14. MACKWORTH-YOUNG , C, SABBAGA, J. and SCHWARTZ, R. S. Idiotypic markers of polyclonal B cell activation: public idiotypes shared by hionoclonal antibodies derived from patients with systemic lupus erythematosus or leprosy. J. Clin. Invest. 79(1987)572-581.
15. MEHRA, V. L., TALWAR, G. P., BALAKRISHNAN, K. and BHUTANI, L. K. Influence of chemotherapy and serum factors on the mitogenic response of peripheral leucocytes of leprosy patients to phytohaemagglutinin. Clin. Exp. Immunol. 12(1972)205-213.
16. NATH, I. Immunology of human leprosy-current status. Lepr. Rev. Special Issue (1983)31s-45s.
17. NATH, I., NARAYANAN, R. B., MEHRA, N. K., SHARMA, A. K. and GUPTE, M. D. Concanavalin A induced suppressor activity in human leprosy. J. Clin. Lab. Immunol. 2(1979)319-324.
18. NATH, I. and SINGH, R. The suppressive effect of M. leprae on the in vitro proliferative responses of lymphocytes from patients with leprosy. Clin. Exp. Immunol. 41(1980)406-414.
19. NATH, I., VAN ROOD, J. J., MEHRA, N. K. and VAIDYA, M. C. Natural suppressor cells in human leprosy: the role of HLA-D identical peripheral lymphyocytcs and macrophages in the in vitro modulation of lymphoproliferativc responses. Clin. Exp. Immunol. 42(1980)203-210.
20. PRYJMA, J., MUNOZ, J., GALBRAITH, R. M., FUDENBERG, H. H. and VIRELLA, G. Induction and suppression of immunoglobulin synthesis in cultures of human lymphocytes: effects of pokeweed mitogen and Staphylococcus aureus Cowan I. J. Immunol. 124(1980)656-661.
21. RAWLINSON, W. D., BASTEN, A. and HARGRAVE, J. C. Clinical significance of changes in serum proteins, immunoglobulins and autoantibodies in leprosy. Int. J. Lepr. 55(1987)277-285.
22. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-267.
23. SAIKI, O. and RALPH, P. Induction of human immunoglobulin secretion. I. Synergistic effects of B cell mitogen Cowan I plus T cell mitogens or factors. J. Immunol. 127(1981)1044-1047.
24. VOLLER, A., BIDWELL, D. E. and BARTLETT, A. Enzyme immunoassays in diagnostic medicine. Theory and Practice Bull. WHO 53(1976)55-56.
1. Ph.D., Department of Biochemistry; All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India.
2. M.D., Department of Dermatovenereology; All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India.
3. M.D., M.R.C.P., Department of Pathology and Department of Biotechnology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India.
4. M.D., Department of Dermatology, Safdarjang Hospital, New Delhi 110029, India.
Reprint requests to Chandri N. Yandava. Ph.D., Division of Tumor Virology, Dana-Farber Cancer Institute, 44 Binncy Street, Boston, Massachusetts 02115, U.S.A.
Present address for Dr. Sharma is Department of Dermatology, Ram Manohar Lohia Hospital, New Delhi, India.
Received for publication on 30 January 1989.
Accepted for publication in revised form on 26 April 1989.
* Values of IgG levels in response to FSA are presented as geometric means with standard deviations. Thus, for example, values with the standard deviation in tuberculoid leprosy (TT) patients group (geometeric mean = 777 x ÷ standard deviation 4.2 ng/ml) would lie between 194.5 and 3263.4 ng/ml (777 ÷ 4.2 and 777 x 4.2 ng/ml).