- Volume 57 , Number 3
- Page: 641–6
Serum lactate dehydrogenase in murine leprosy: source and isozymes
ABSTRACT
The bulk of the lactate dehydrogenase (LDH) that increases in the serum of mice infected with Mycobacterium lepraemurium (MLM) derives f rom the liver and corresponds to the isozyme V. MLM-induced granulomas continuously arise in the liver and steadily increase in size until the animal's death. Growing granulomas push the adjacent hepatocytes away and cause them to disrupt and to release their cytoplasmic contents, including LDH. The LDH is then picked up by the infiltrating phagocytes and/ or admixed with the circulating blood. Other LDH-containing organs (including the testis with its additional isozyme LDH-X) in the infected or normal animals do not seem to significantly contribute to the serum levels of LDH.The study of the liver-associated histochemical and biochemical changes in this controlled model of murine leprosy allows us to gain insight into the overall pathology of this mycobactcriosis. In some respects this sheds light on the liver involvement in human leprosy; a subject on which results of all sorts have been published.
RÉSUMÉ
La plus grande partie de l'élévation constatée dans les taux de lactate dehydrogenase (LDH) dans le sérum de souris infectées par Mycobacterium lepraemurium (MLM) provient du foie et correspond à l'isozyme V. Des granulomes induits par la présence de MLM surviennent continuellement dans le foie; leur volume s'accroît régulièrement jusqu'à la mort de l'animal. Lors de leur croissance, les granulomes repoussent les hepatocytes adjacents et entraînent leur dislocation ainsi que la libération de leur contenu cytoplasmiquc, y compris la LDH. La LDH alors est soit reprise par les phagocytes d'infiltration, soit pénètre dans le sang circulant, les deux mécanismes pouvant se combiner. D'autres organes contenant de la LDH (y compris le testicule, avec son isozyme supplémentaire LDH-X) ne paraissent pas, tant chez l'animal infecté que chez l'animal normal, contribuer significan vement aux taux sériques de LDH.L'étude des modifications histochemiques et biochemiques du foie, dans ce modèle contrôlé de la lèpre murine, permet d'obtenir un aperçu de la pathologie globale de cette mycobactériosc. A certains égards, ces observations éclairent le mécanisme de l'atteinte du foie au cours de la lèpre humaine, un sujet sur lequel toutes sortes de résultats ont été publiés.
RESUMEN
Prácticamente la totalidad de la deshidrogenasa del ácido láctico (LDH) que se incrementa en el suero de los ratones infectados con el Mycobacterium lepraemurium (MLM) deriva del higado y corresponde a la isoenzima V. Los granulomas inducidos por el MLM, aparecen en el higado y crecen sostenidamente hasta que sobreviene la muerte del animal. Los granulomas en crecimiento empujan a los hepatocitos adyacentes y ocasionan su ruptura. Los hepatocitos dañados liberan su contenido citoplásmico, incluyendo a la LDH, el cual es recogido por los fagocitos infiltrantes o por la circulación sanguínea. Otros órganos en los animales infectados o control, no parecen contribuir significativamente a los niveles séricos de la LDH. El estudio de los cambios histoquímicos y bioquímicos asociados al hígado en el modelo de la lepra murina, además de que permite ahondar en el conocimiento de esta micobacteriosis, ayuda, en cierto modo, a ordenar ideas sobre la patología hepática en la lepra de los humanos; aspecto sobre el que se han publicado resultados de todo tipo.The liver is a target organ in human and murine leprosy. Associated with liver involvement are pathological and biochemical changes, the first being more constant than the second (7,8). Within the liver-associated biochemical changes in human leprosy, alterations in the levels of glutamicpyruvic and glutamic-oxalacetic transaminases (GPT and GOT) and lactate dehydrogenase (LDH) have been recognized (2,6). As expected, variations attributable to the inherent heterogeneity of the patients have led to results similarly heterogeneous such that leprosy-related patterns of serum GOT, GPT, and LDH do not exist. In the more controllable model of murine leprosy, serial changes in those enzyme activities appear and are proportional to the degree of liver involvement. It has been noted that Mycobacterium lepraemurium (MLM)-infected animals show progressive increments in their serum levels of GPT, GOT and LDH (3,10) that are measurable far in advance of the earliest signs of infection. Although the increments in the levels of transaminases appeared earlier than the increments in LDH levels (1 month versus 2 months, on the average), the LDH increments were more marked than the transaminase increments. The same situation was found in the nine-banded armadillo (Dasypus novemcinctus) infected with M. leprae (11); in addition, an obvious parallelism between increased levels of serum LDH and antibodies to M. leprae was also observed (9). We felt that by monitoring LDH levels in infected versus control animals, at periodic intervals, it might be possible to identify those animals with an ongoing infection. This has proved to be the case.
Although the liver is not the only LDH-containing organ affected by MLM infection, in the present study we present evidence that indicates that the liver is practically the only source of the LDH isozyme V that increases in the sera of the infected mice.
MATERIALS AND METHODS
Mice and bacilli. Three-month-old, male and female NIH mice were inoculated intraperitoncally (i.p.) with 108 M. lepraemurium Hawaiian strain. Other data in relation to this point have been given elsewhere (10).
LDH and isozymes. Quantitation of LDH activity and LDH-isozymes in serum and tissue extracts were determined by using the Lakeside LDH-UV system commercial kit (Boehringer, Mannheim, West Germany) and the method of Mager, et al. (5), respectively. Briefly, isozymes were separated by electrophoresis on cellulose membranes (Beckman Instruments, Inc., Fullerton, California, U.S.A.) with a barbital buffer (μ = 0.5, pH 8.6) as the electrode solution for 55 min at 200 V. The membranes were then placed on top of 7.5 x 5 cm glass slides containing 5.0 ml of gel substrate. Each 5.0 ml of the gel substrate contained 75 mg agarose in 3.5 ml of 0.2 M Tris-HCI (pH 8.3), 0.1 ml of 60% syrup sodium lactate, 1.0 mg nitroblue tetrazolium (NBT) in 1.0 ml distilled water (DW), 0.1 mg phenazine metasulfate (PMS) in 0.1 ml DW, and 5.0 mg NAD+ in 0.3 ml DW. All chemicals were from Sigma Chemical Co., St. Louis, Missouri, U.S.A. The electrophoresis membrane was maintained on top of the gel substrate for 30 min at 37ºC in a moist chamber protected from light. The reaction was stopped by immersing both the gel and the membrane in 5% acetic acid. After drying, the membrane was read in a densitometer set at 500 nm, and the areas under the curve were recorded.
Tissue extracts were prepared in the cold from the liver, spleen, testis, brain, heart, and kidney by homogenization in a glass tissue grinder in the proportion of 1 g tissue/ 4 ml normal saline. After centrifugation, the supernatants were diluted as required.
Histochemical demonstration of LDH. This was performed in 6-μm frozen liver and spleen sections. After cutting in a cryostat (Tissuetck II; Miles Laboratories, Inc., Elkhart, Indiana, U.S.A.), the sections were fixed for 5 min with 1.25% glutaraldehyde in 0.15 M PBS (pH 7.2), washed twice in PBS, and stained in the dark with a solution containing 17.5 ml of 0.15 M Tris-HCI (pH 8.3), 0.5 ml of 60% syrup sodium lactate, and 5 mg in 0.5 ml each of NBT, PMS, and NAD+. After rinsing in water and air drying, the slides were stained by the Ziehl-Neelsen method without the blue counterstain. The dried sections were immersed in xylene for 2 min and then mounted in synthetic resin dissolved in xylene.
RESULTS
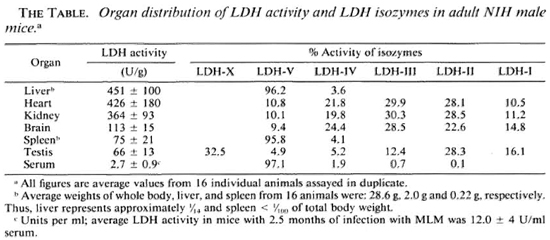
Serum LDH and isozymes. In order to identify the isozymes and the source of the LDH present in the sera of the normal and infected mice, LDH activity and isozymes were analyzed in saline extracts of several organs. Figure 1 shows a representative result of the distribution of the isozymes in several of the organs tested. The kidney, heart, and brain, and testis to a lesser degree, contained variable amounts of all of the five known isozymes; the testis also contained isozyme X and the spleen and the liver contained over 95 % isozyme V and traces of the other isozymes (mainly isozyme III). Figure 1 also shows the electrophoretic mobility of serum LDH in normal (track 5) and MLM-infected (track 4) animals. It can be seen that in the infected mouse (5 months of infection) the increment in LDH activity is limited to those isozymes present in the normal liver and serum, that is, no other normal or abnormal isozymes increased in the sera of the infected animals. The Table shows the amounts of total LDH activity (U/g of tissue or U/ml of serum) and the percent distribution of those isozymes found. These data indicate that: a) the liver, spleen, and serum contain LDH; b) in all three cases, over 95 % is isozyme V; c) the liver contains around six times more LDH than the spleen (451 ± 100 U/g vs 75 ± 21 U/g); and d) while the liver represents approximately 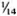 (
(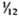 to
to 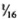 ) of the body weight in the mouse, the spleen accounts for less than
) of the body weight in the mouse, the spleen accounts for less than 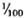 (
(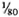 to
to 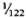 ). Thus, the liver is by far the most important source of LDH isozyme V (LDH-V) that increases in the sera of MLM-infected mice.
). Thus, the liver is by far the most important source of LDH isozyme V (LDH-V) that increases in the sera of MLM-infected mice.
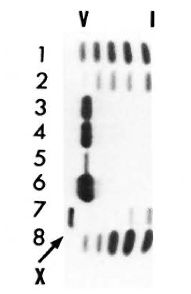
Fig. 1. LDH isozymes in the heart (1), brain (2), spleen (3), liver (6), testis (7). and kidney (8) from normal NJH mice (a representative result). Track 4 = serum from an animal with 5 months of MLM infection; tract 5 = serum from a normal mouse.
The above data also show the lack of testicular LDH isozyme X (LDH-X) in the sera of normal and infected animals. The histopathological study of the testis in mice with more than 4 months of infection showed no abnormal changes in the testicular tissue, with only small lepromas being seen in the tunica albuginea, the connective tissue structure enclosing the testis. Accordingly, periodic determination (32 to 144 days postinoculation) of LDH-X in testicular extracts of MLM-infected mice showed no abnormal LDH activities, i.e., all of the values fell within the limits of 20 ± 10 U/g found in the control animals.
Figure 2 shows the normal distribution of LDH-V in the mouse's hepatocytes. Although cytoplasmic, most of the activity is located around the cellular nuclei. Figure 3 illustrates how a growing (still small but highly bacilliferous) granuloma pushes away the adjacent hepatocytes, leading to their disruption. The disrupted cells release LDH and other cytoplasmic constituents into the surrounding area and from there to the circulation. Figure 4 shows another granuloma surrounded by disorganized hepatic tissue where many hepatocytes appear damaged and contain only traces (if any) of LDH activity. Figure 4 also shows a central MLM-loaded macrophagic core and peripheral, nonparasited cell infiltration. The small amount of LDH activity sometimes present in bacilli-containing macrophages does not seem to be a meaningful source of LDH activity.
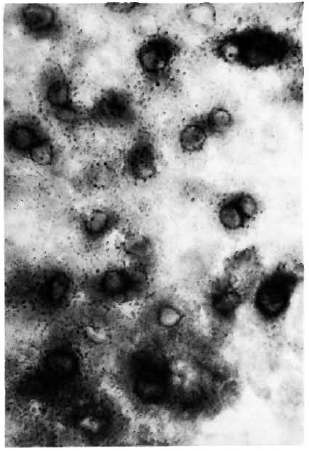
Fig. 2. Typical LDH distribution in the liver of a normal mouse. In this histochemical study of the liver, the enzyme is allowed to act on the lactate and in so doing reduces the coenzyme NAD to NADH,. At least one other enzyme (probably of perinuclear distribution) is required to transfer the hydrogen from NADH, to the tetrazolc; the blue formazan is deposited by this enzyme, not by the LDH (6-μm liver section stained for LDH with no counterstain x 500).
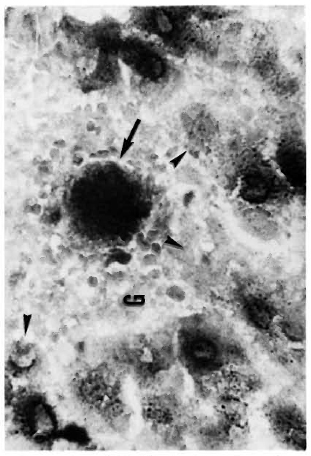
Fig. 3. A small MLM-induced granuloma (G) in the liver of a mouse with 4 months of infection. One or a few highly parasitized macrophages are seen at the center of the granuloma ( ). The growing granuloma pushes away the adjacent parenchymal cells leading to their destruction (
). The growing granuloma pushes away the adjacent parenchymal cells leading to their destruction ( ) (tissue section stained for LDH and AFB with no counterstain x500).
) (tissue section stained for LDH and AFB with no counterstain x500).
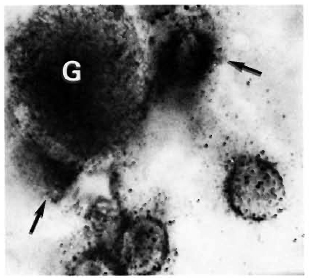
Fig. 4. A small, growing, bacilliferous macrophagic granuloma (G) in the process of destroying adjacent hepatocytes. Disrupted cells (
 ) release their contents (including LDH) into the surrounding medium (tissue section stained for LDH and AFB with no countcrstain; oil immersion x 1250).
) release their contents (including LDH) into the surrounding medium (tissue section stained for LDH and AFB with no countcrstain; oil immersion x 1250).
DISCUSSION
The liver has long been recognized as a target organ in human leprosy. The presence of periportal lepromas, the bacillary parasitation of Kupfler's and endothelial cells, and the lack of involvement of the hepatocytes, are all well documented. Liver involvement in leprosy has motivated the study of its function by several groups. In general terms, although severe hepatic dysfunction has not been found in nonrcactional leprosy, important alterations associated with increments in the GOT and GPT levels in reactional leprosy have been reported (1). Results on the levels of LDH in leprosy have varied. The most consistent results indicate alterations in the serum levels of LDH isozymes IV and V. However, these isozymes have been found to be increased, diminished, and even within the normal range (12). Alterations in the serum levels of LDH and LDH isozymes have not been found to be related to a particular form of leprosy or to the clinical status of the disease (quiescent, active or reactional). This heterogeneity of results corresponds, we believe, to the inherent heterogeneity of the individuals studied. Since in murine leprosy the liver is also involved and similar histopathological and biochemical changes occur (3,10,14), we thought of the study of this mycobacteriosis as a more controllable model to gain insight into what may happen in human leprosy.
The biochemical quantitation of LDH and LDH isozymes in normal serum and tissue extracts, and the assessments of the particular contribution of several organs to the serum LDH activity, clearly indicate that the liver is the main, if not the only, source of the LDH (LDH-V) that increases in the sera of mice infected with MLM. This is corroborated by the histochemical study of the liver: as the infection goes on, granulomas (mostly macrophagic during the first two thirds of the infection time, and macrophagic with an outer sheet of neutrophils during the last third of it) appear and increase both in size and number, pushing away and disrupting the adjacent hepatocytes. The disrupted hepatocytes release their cytoplasmic contents, including LDH. The few formazan granules observed in the granulomas may represent: a) LDH activity originally present in the hepatocytes whose debris has been taken up by the phagocytes, b) real LDH activity, or c) nonLDH, tctrazole (NBT)-reducing activity.
On the other hand, it has recently been reported that cultivable mycobacteria contain LDH (4) with LDH zymograms specific for each mycobacterium (M. avium, M. tuberculosis, M. scrofulaceum) and no strain variation within the same species (13). M. leprae grown in armadillo liver has also been found to contain its own LDH (15); the amount of LDH in M. leprae was approximately  of that found in normal liver, and M. leprae-infected liver contained less LDH than that found in the normal organ. So far, there is no information on the presence and amounts of LDH in MLM but, from our results, it would seem that neither the macrophage-loading bacilli nor the bacilli-containing phagocytes are meaningful sources of the LDH that increases in the serum of the MLM-infected mouse. The search for MLM-specific LDH isozymes is, however, a task that must be accomplished both to complement the study of this mycobactcrium and to assess their exact contribution (if any) to the whole increment of LDH in the serum of MLM-infected animals.
of that found in normal liver, and M. leprae-infected liver contained less LDH than that found in the normal organ. So far, there is no information on the presence and amounts of LDH in MLM but, from our results, it would seem that neither the macrophage-loading bacilli nor the bacilli-containing phagocytes are meaningful sources of the LDH that increases in the serum of the MLM-infected mouse. The search for MLM-specific LDH isozymes is, however, a task that must be accomplished both to complement the study of this mycobactcrium and to assess their exact contribution (if any) to the whole increment of LDH in the serum of MLM-infected animals.
Acknowledgments. The authors are indebted to Q.B.P. Antonina Oltra, Q.B.P. Patricia Méndez A., and Dr. S. Estrada-Parra for their help in different aspects of the study. Financial support came from the D.E.P.I., I.P.N., to the project "Consecuencias bioquímicas de la infección leprosa en el raton" (862193/862196). J. Luna holds a fellowship from COSNET and O. Rojas-Espinosa is a fellow from COFAA and SNI, Mexico.
REFERENCES
1. BALAKRISHNAN, S. Biochemical aspects of reactional states in leprosy. Lepr. India 48(1976)406-412.
2. GARCIA, R., SALIBIAN, A., LLÓRENTE, B., PACIN, A. and FLIESS, E. L. Perfil de las isoenzimas de la deshidrogenas láctica en el suero de pacientes hansenianos. Hansen Int. 5(1980)119-122.
3. HA, D. K. K., LAWTON, J. W. M. and GARDNER, I.D. Experimental murine leprosy: a biochemical study emphasizing lysosomal enzyme changes in vivo and enzyme secretion by macrophages in vitro. Exp. Mol. Pathol. 40(1984)177-194.
4. KATOCH, V. M., SHARMA, V. D., DATTA, A. K., SHIVANNAVAR, C. T., KATOCH, K., KANNAN, K. B. and BHARADWAJ, V.P. Isoenzymes of mycobacteria-II. Relevance of LDH zymograms in taxonomy and identification. Indian J. Lepr. 57(1985)107-114.
5. MAGER, M., BLATT, W. F. and ABELMANN, W. H. The use of cellulose acetate for the clcctrophoretic separation and quantitation of serum lactic dehydrogenase isozymes in normal and pathologic states. Clin. Chim. Acta 14 (1966) 689-697.
6. PACIN, A. and FLIESS, E. L. Estudios enzimáticos en pacientes hansenianos. 1. Actividad de las transaminasas glutámico-piruvica y glutámico-oxalacctica. Hansen Int. 3(1978)151-159.
7. PACIN, A., FLIESS, E. L. and LLORENTE, B. E. La función hepática a traves del espectro clinico de la hanseniasis. Hansen Int. 5(1980)93-111.
8. POWELL, C. S. and SWAN, L.L. Leprosy: pathologic changes observed in 50 consecutive necropsies. Am. J. Pathol. 31(1955)1131-1147.
9. ROJAS-ESPINOSA, O., MENDEZ, P., OLTRA, A. and ARCE, P. Antimycobacterial antibodies in Dasypus novemcinctus infected with Mycobacterium leprae and their correlation with the serum levels of lactate dehydrogenase. Lepr. Rev. 57(1986)317-327.
10. ROJAS-ESPINOSA, O., OLTRA, A., ARCE, P. and MENDEZ, I. Serum enzymatic changes following infection of mice with Mycobacterium lepraemurium. Int. J. Lepr. 53(1985)258-261.
11. ROJAS-ESPINOSA, O., QUESADA, P. F., OLTRA, A., ARCE, P., ESTRADA, P. S. and BUCHANAN, T. M. Biochemical alterations in the serum of armadillos (Dasypus novemcinctus) infected with Mycobacterium leprae; a preliminary report. Int. J. Lepr. 53(1985)262-268.
12. SAOJI, A. M. and KELKAR, S. S. Quantitative studies of serum lactate dehydrogenase isoenzyme patterns in leprosy. Lepr. Rev. 52(1981)309-314.
13. SHARMA, V. D., KATOCH, V. M., DATTA, A. K., SHIVANNAVAR, C. T., KATOCH, K., KANNAN, K. B. and BHARADWAJ, V. P. Isoenzymes of mycobacteria: their role in the taxonomy of mycobacteria with special reference to M. leprae. In: Proceedings of the Indo-UK Symposium on leprosy, Agra, India. April 7-10. 1986. Agra: Central JALMA Institute for Leprosy (ICMR), 1987, pp. 210-218.
14. TANIMURA, T. and NISHIMURA, S. Studies on the pathology of murine leprosy. Int. J. Lepr. 20(1952)83-94.
15. WHEELER, P.R., BHARADWAJ, V.P., KATOCH, V.M. and KANNAN, K.B. Lactate dehydrogenase in Mycobacterium leprae grown in armadillo liver. Int. J. Lepr. 52(1984)371-376.
1. Q. B. P., M. C; Departamento de Inmunología, Escuela Nacional de Ciencias Biológicas, I. P. N., Carpió y Plan de Ayala, Colonia Santo Tomas, 11340 Mexico, D. F., Mexico.
2. Q. B. P., Sc. Dr., Departamento de Inmunología, Escuela Nacional de Ciencias Biológicas, I. P. N., Carpió y Plan de Ayala, Colonia Santo Tomas, 11340 Mexico, D. F., Mexico.
Reprint requests to Dr. Rojas-Espinosa.
Received for publication on 6 February 1989.
Accepted for publication on 25 April 1989.