- Volume 57 , Number 3
- Page: 647–51
Impact of MDT on incidence rates of leprosy among household contacts part 1. baseline data
ABSTRACT
This preliminary study f rom Gudiyatham Taluk, the leprosy control area of the Schieffclin Leprosy Research & Training Centre, investigated the incidence rates of leprosy among household contacts during the period 1970-1985. The incidence rates of leprosy among the household contacts prior to and during the initiation of multidrug therapy are presented here. The overall incidence rate among the household contacts was 4 per 1000 person-years at risk. Contacts of multibacillary and paucibacillary cases had a relative risk of 3-6 times and 2-4 times the risk of leprosy in the general population, respectively. The incidence rates among children were higher than adults; the peak age-specific incidence rate was between 5-9 years, and nearly one third of the primary cases were children. These findings are presented and the methodological issues discussed.RÉSUMÉ
Lors d'une étude préliminaire dans le Gudiyatham Taluk, une région où la lutte contre la lèpre est menée par le Schieffclin Leprosy Research and Training Centre, on a étudié les taux d'incidence de cette maladie parmi les contacts domiciliarios au cours de la période 19701985. On fournit ici les chiffres des taux d'incidence de la lèpre chez les contacts domiciliaires avant le début de la polychimiothérapic, de même qu'au cours de la période où cette méthode de traitement a été utilisée. Le taux d'incidence global parmi les contacts domiciliaires a été de 4 par 1000 personnes/année à risque. Les contacts de cas multibacillaires ou paucibacillaires avaient respectivement un risque relatif de 3 à 6 fois plus élevé et de 2 à 4 fois plus élevé, que celui présenté par la population générale. Les taux d'incidence parmi les enfants étaient plus élevés que chez les adultes. Le pic des taux d'incidence spécifique pour l'âge se situait entre 5 et 9 ans. Prés du tiers des cas primaires ont été observés chez les enfants. Ces observations sont présentés, et les problèmes méthodologiques qu'elles soulèvent sont discutés.RESUMEN
En osle estudio preliminar efectuado en Gudiyatham Taluk, el área de control del Centro Schicífelin para investigación y entrenamiento sobre la lepra, se investigó la incidencia de la enfermedad entre los contactos familiares durante el período de 1970 a 1985. Aquí se presenta la incidencia de la enfermedad entre los contactos familiares antes y durante el tratamiento de los pacientes con múltiples drogas. La incidencia global fue de 4 por 1000 personas-año en riesgo. Los contactos de los casos multibacilares y paucibacilares tuvieron, respectivamente, un riesgo relativo 3-6 veces y 2-4 veces mayor que el riesgo mostrado por la población general. La incidencia entre los niños fue mayor que entre los adultos; el pico de incidencia en relación a edad estuvo entre los 5 y 9 años y casi un tercio de los casos primarios fueron niños. Se presentan y discuten estos hallozgos y los aspectos metodológicos del estudio.The revised strategy based on multidrug therapy (MDT) as suggested by the World Health Organization (WHO) (12) is now accepted as our greatest hope to control leprosy in a time-bound period. The difference in impact on infectivity when patients are put on MDT, as compared with monotherapy, is largely due to the rapid killing of the bacilli with the use of rifampin. The reduction in infectivity should lead to a decrease in transmission, thereby resulting in a decline of leprosy incidence rates.
It has been well established that household contacts have a higher risk of developing leprosy than noncontacts (1-4, 6-10,13). The impact of MDT on incidence rates (IR) should first be reflected in a decrease in IR among household contacts.
Two previous studies (6,8) have been done using data from Gudiyatham Taluk, the leprosy project area of the Schieffelin Leprosy Research and Training Centre, Karigiri, South India. In the first paper, based on 5088 cases and 22,652 household contacts 8), the incidence rate of leprosy per 1000 household contacts during 1962-1970 was calculated to be 6.8. The second study was based on a sample of 1564 primary cases and 9162 household contacts covering the period 1962-1975 (6); the overall IR of leprosy among household contacts was 3.8 per 1000 person-years at risk (PYR). MDT was introduced in this area during 1982-1983.
In this paper, the incidence rates of leprosy for 1970-1985, prior to and during the initiation of MDT, are presented based on about 9000 primary cases and 40,000 household contacts, with a total of 180,000 PYR. A subsequent paper will evaluate time trends and the impact on transmission within households, if any, after the implementation of MDT.
MATERIALS AND METHODS
The study area, Gudiyatham Taluk, situated in the northwestern part of North Arcot District in Tamil Nadu, South India, was described earlier (8). The estimated midyear population in 1962 was 390,000, which grew to 517, 400 in 1985. The leprosy control program was started in 1962 and opcrated 44 decentralized monthly clinics. These roadside clinics were distributed based on terrain, patient density and accessibility, so that the patients did not have to travel more than 8-10 km to come to a clinic. A mobile team visits the clinics and provides comprehensive health care. Contact surveys are done once a year by paramedical workers under supervision. Information on the enumeration and examination of the contacts are recorded on contact survey registers.
Household contacts of leprosy cases registered for treatment during the period 19701985 formed the main study group. Multibacillary (MB) patients registered prior to 1970, but who were still under treatment, and their household contacts are also included.
A primary case is defined as the case of leprosy who was alive when the family came under surveillance and who was probably responsible for the introduction of leprosy within the household (6). The primary case was identified using a flow chart, taking into consideration the age, classification, deformity, and duration of the disease among all the cases identified during the contact survey.
The secondary cases within a household were divided into two groups: a) those detected with leprosy during the first contact survey--these cases were labeled as coprcvalent cases; and b) those cases which arose from household contacts who were examined once and declared healthy-these were considered as true incident cases of leprosy and were used to compute incidence rates of leprosy among household contacts.
A household (family) was defined as individuals living together under the same roof and partaking of food from the same kitchen. Familial and nonfamilial individuals living together for more than 6 months were included. Child contacts were defined as contacts below the age of 15 years.
Person-years at risk (PYR) was computed for each of the household contacts using specific guidelines. In general, contacts seen twice, 1 year apart, were given 1 PYR. Those seen only once were excluded from this calculation.
RESULTS
The age, sex, and type of disease distribution of the primary cases are given in Table 1. Of the 8642 primary cases, 5052 (58.5%) were male; 2984 (34.5%) were below 15 years ofage; 7181 (83.1%) were classified as paucibacillary (PB) cases.
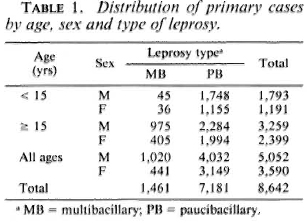
The distribution of the 40,625 contacts and their person-years of follow up are shown in Table 2 by age, sex, and type of primary cases. Of the 40,625 household contacts, 24,101 (59.3%) were male and 15,158 (37.3%) were below the age of 15 years at the time of entry into follow up.
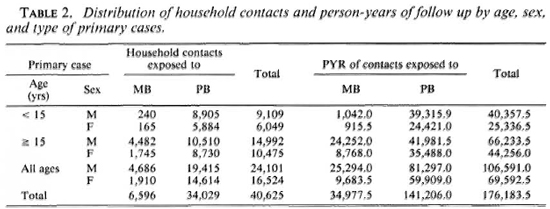
Among the 40,625 contacts there were 1225 co-prevalent cases of leprosy; 715 developed leprosy during follow up, and only these are considered true incident cases. The 40,625 contacts (excluding co-prevalent cases and those seen only once) contributed a total or 176,183.5 PYR. Thus, the overall incidence rate is 4.06 per 1000 PYR among household contacts of all types of leprosy cases.
The distribution of the 715 incident cases by age at detection, sex, and type of the leprosy is given in Table 3. Males and females are equally divided among the 715 incident cases; 429 (60%) cases were less than 15 years of age. The mean (± S.D.) ages of male and female incident cases were 17.8 (± 14.6) and 17.7 (± 14.2), respectively, the difference not being statistically significant. Of the 715 incident cases, 680 (95.1%) were paucibacillary, 30 (4.2%) were multibacillary, and 5 (0.7%) cases could not be classified.
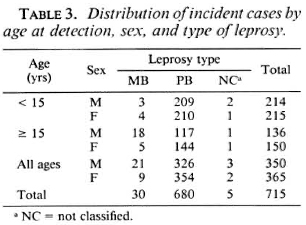
The age-sex specific incidence rates (per 1000 PYR) among household contacts are shown in Table 4 and The Figure. The peak incidence rate among males was between 10 to 14 years, closely followed by 5 to 9 years. Among females, the peak incidence is in the age group 5-9 years.
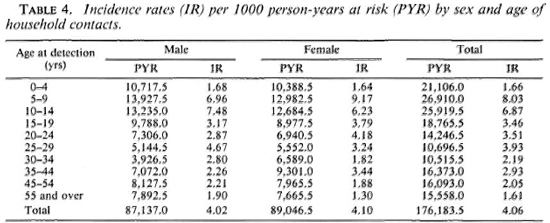
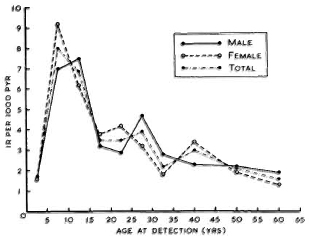
The figure. Incidence rates by sex and age of household contacts.
The incident cases and the incidence rates (per 1000 PYR) in relation to age, sex, and type of leprosy in the primary cases are presented in Table 5. Contacts of male and female primary cases had an incidence rate of 4.14 and 3.94 per 1000, respectively, the difference not being statistically significant. The incidence rate in contacts of adult primary cases was 4.62 per 1000 PYR as compared to 3.12 per 1000 PYR in those exposed to a child primary case; this difference was statistically significant (p < 0.01). The contacts of MB cases were at a significantly higher risk (6.40 per 1000 PYR) compared to PB contacts (3.48 per 1000 PYR).
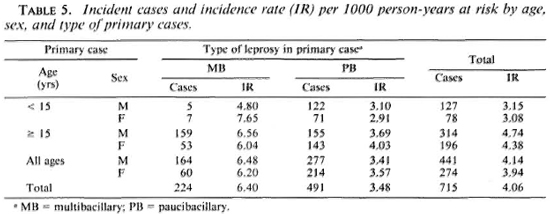
Child contacts in general had a statistically significant higher incidence rate than adult contacts with respect to the characteristics of the primary cases. Child contacts of MB cases had a significantly higher incidence rate of 8.45 per 1000 PYR compared with 5.02 per 1000 PYR for child contacts of PB cases (p < 0.01).
DISCUSSION
The incidence rates for leprosy among household contacts is an important epidemiological indicator to monitor changes in the character and trends of the disease. However, before concluding that the changes observed over time are due to the effect of control programs, other reasons, such as differences in methodology of measurement or definitions, should be ruled out. Thus, the comparison between MDT and dapsone monotherapy in the transmission of the disease in households should first equalize the control measures adopted and the effort expended in both regimens not only in households with known leprosy cases but also in the rest of the endemic community where unknown cases may also abound. Fortunately, data on these operational aspects are available for this study population.
Variation in the definition of a "primary" case or "index" case as well as the definition of an "incident" case could itself produce changes in the incidence rates. Several problems, such as recall bias, migration of an index case, or break up of joint families, are inherent in identifying an "index" case from whom other members in a family (or household) could have aquircd the disease. Further, in a hyperendemic area where clusters of related households live together within a circumscribed geographic area, the accurate identification of the true index case becomes difficult, if not impossible. The implication of such inaccuracies on the calculation of incubation periods or durations of follow up must be considered. In this paper, instead ofindex cases, the most probable primary case was used.
Inclusion of all "secondary" cases in a family presents another reason for differences in the reported incidence rate among household contacts. In some of the earlier studies (2), the incident and primary cases were differentiated using historical information. In this study, only those contacts who were found healthy at first examination and then developed leprosy were included under "incident" cases.
Allowing for these methodological variations, the incidence rate in Gudiyatham Taluk during 1975-1985 was 4 per 1000 PYR among the household contacts. As in previous studies (2,3,6,8), contacts of MB cases show a significantly higher incidence of leprosy compared with contacts of PB cases. This is not surprising, since MB cases excrete larger numbers of bacilli (11,12). Compared to the incidence rates of leprosy among the general population (5), the household contacts of MB cases have a relative risk of 3-6 times and household contacts of PB cases a relative risk of 2-4 times. The incidence rate among child contacts was significantly higher than among adult contacts. The age-specific incidence rates were consistent with findings of earlier studies, with a peak between age 5 and 9 (6,8). Further analysis is being done in relation to age-specific shifts in MB and PB incidence rates, time cohorts and age cohorts, and comparison with changes in incidence rates in the same area.
Nearly a third of the primary cases were children. The role of children getting infected and subsequently spreading the disease within their homes needs further investigation.
The earlier paper (6) dealt with the problem of community sources of infection maintaining a high incidence rate among contacts, even after the infectivity of the primary case decreases. An age-specific shift to the right, in the incidence rate among household contacts, will probably occur only after a similar shift has occurred in the age distribution of the primary cases.
Acknowledgments. We wish to thank Mr. J. Samuel and the staff of the Department of Epidemiology, Karigiri, for their involvement in data collection. We also wish to thank the staff of the Department of Biostatistics. Christian Medical College, Vellore, for their help in data entry and analysis, and Mr. Lewis Kumar, Mr. Krishnan, and Miss Shanthi for secretarial help.
REFERENCES
1. COCHRANE, R. G. and RAJAGOPLAN, L. M. P. The study of family susceptibility in relation to the epidemiology of leprosy. Lepr. India 15(1943)76-81.
2. DOULL, J. A. The importance of field studies of leprosy with special reference to the risk of household exposure. Am. J. Hyg. 29(1939)27-33.
3. FIGUEREDO, N. and BALAKRISHNAN, V. Risk of infection in leprosy. Lepr. Rev. 38(1967)87-92.
4. GANAPATI, R., REVANKAR, C. R., CHRISTIANA and ROMANO. Associated cases in the families of school children with leprosy. Lepr. Rev. 49(1978)43-46.
5. JESUDASAN, K., BRADLEY, D., SMITH, P. G. and CHRISTIAN, M. The effect of intervals between surveys on the estimation of incidence rates of leprosy. Lepr. Rev. 55(1984)353-359.
6. JESUDASAN, K., BRADLEY, D., SMITH, P. G. and CHRISTIAN, M. Trends in the analysis of incidence rates of leprosy among household contacts. Indian J. Lepr. 56(1984)792-806.
7. RAMU, G., MALAVIYA, G. N., BHARADWAJ, V. P., SENGUPTA, V., SINHA, S., RAMANATHAN, V. D., PAL, C. and DESIKAN, K. V. Studies on healthy contacts of leprosy patients; a preliminary report. Indian J. Lepr. 57(1985)796-803.
8. RAO, P. S. S., KARAT, A. B. A., KALIAPERUMAL, V. G. and KARAT, S. Transmission of leprosy within households. Int. J. Lepr. 43(1975)45-54.
9. RASI, E., CASTELLAZZI, Z., GARCIA, L., QUEVEDO, L. and CONVIT, J. Evaluation of "chemical isolation" in 1, 168 leprosy patients' homes. Int. J. Lepr. 43(1975)101-105.
10. SHARMA, V. K. The epidemiologic significance of leprosy within the household. Int. J. Lepr. 36(1968)1-16.
11. WATERS, M. F. R. Leprosy. Br. Med. J. 283(1981)1320-1322.
12. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
13. WORTH, R. M. and WONG, K. O. Further notes on the incidence of leprosy in Hong Kong children living with a lepromatous parent. Int. J. Lepr. 39(1971)745-749.
1. D.P.H., Professor and Head; Christian Medical College, Vellore 632002, India.
2. B.Sc., Statistical Assistant, Department of Biostatistics, Christian Medical College, Vellore 632002, India.
3. Ph.D.; Department of Epidemiology and Leprosy Control, Schieffelin Leprosy Research and Training Centre, Karigiri 632106, India.
4. M.B.B.S., D.T.M.&H., Dip.Epid. (Prague), Director and Chief, Department of Epidemiology and Leprosy Control, Schieffelin Leprosy Research and Training Centre, Karigiri 632106, India.
Received for publication on 29 September 1988.
Accepted for publication in revised form on 7 March 1989.