- Volume 57 , Number 3
- Page: 652–8
Identification of Mycobacterium leprae antigens in tissues of leprosy patients using monoclonal antibodies
ABSTRACT
Five monoclonal antibodies (MAbs) directed against antigens of Mycobacterium leprae were tested for their ability to bind to components of tissue sections prepared f rom biopsies taken f rom patients with various forms of leprosy. Immunoperoxidase was the most successful marker system used, although immunofluorescence and alkaline phosphatase were also successful in certain cases. Positivity was high with all five antibodies successfully staining those sections containing a bacterial index of 3+ or more; sections with 0 bacterial counts also had areas staining positively with two of the MAbs. The positive staining in the tissues was confined to areas infiltrated by inflammatory cells; however it was not identifiable as being associated with individual bacteria. These findings suggest that immunostaining with specific monoclonal antibodies can help to identify leprosy in diagnostic samples in which acid-fast bacilli are not identifiable by standard histochemical means. Immunohistochemical techniques are likely to be valuable in studies of the distribution of M. leprae antigens and their association with individual tissue elements.RÉSUMÉ
On a étudié 5 anticorps monoclonaux (MAbs) dirigés contre les antigènes de Mycobacterium leprae, quant à leur capacité de se lier aux constituants de coupes tissulaires préparées à partir de biopsies obtenues chez des malades atteints de différentes formes de lèpre. Parmi les systèmes marqueurs utilisés, l'immunoperoxytlase était le meilleur. Néanmoins, la phosphatase alcaline, de môme que l'immunofluorescence, pouvaient également produire des résultats dans certains cas. Un indice élevé de positivité a été observé pour chacun des 5 anticorps, permettant de colorer les sections tissulaires ayant un indice bactériologique de 3 + ou davantage. Les coupes bactériologiquement négatives présentaient cependant également des zones qui pouvaient être colorées positivement par deux de ces anticorps monoclonaux. La coloration positive dans les tissues était limitée aux régions infiltrées par des cellules inflammatoires. Toutefois, il n'était pas possible d'identifier ces colorations au niveau de bactéries individuelles. Ces observations suggèrent que l'immunocoloration par des anticorps monoclonaux spécifiques, pourrait être utilisée pour identifier la lèpre dans des échantillons diagnostiques, alors que les bacilles acido-résistants ne peuvent pas être mis en évidence par les méthodes histochimiques actuelles. Des techniques immunohistochimiques se révéleront vraisemblablement plus utiles pour étudier la distribution des antigènes de M. leprae, de même que leur association avec des éléments individuels dans les tissus.RESUMEN
Se probaron 5 anticuerpos monoclonalcs (AcsM) dirigidos contra antígenos del Mycobacterium leprae en cuanto a su capacidad para unirse a componentes tisulares de secciones preparadas a partir de biopsias de pacientes con varias formas de lepra. El sistema de la inmunoperoxidasa fue el más exitoso, la inmunofluorescencia y. el sistema de la fosfatasa alcalina resultaron útiles en ciertos casos. La positividad fue alta con los 5 anticuerpos, sobre todo en secciones con un índice bacteriológico de 3+ ó más; las secciones con cuentas bacterianas de cero también tuvieron áreas con tinción positiva en el caso de 2 de los AcsM. La tinción positiva en los tejidos estuvo confinada a áreas infiltradas por células inflamatorias pero no pareció estar asociada a bacterias individuales. Estos hallazgos sugieren que la tinción con anticuerpos monoclonalcs específicos, puede ayudar a identificar evidencias de la lepra en secciones preparadas con fines diagnósticos en las cuales no se observan bacilos ácido resistentes por los métodos histoquímicos convencionales. Las técnicas inmunohistoquímicas son de gran valor en el estudio de la distribución de los antígenos del M. leprae y en el estudio de su asociación con elementos tisulares individuales.Tissues invaded by Mycobacterium leprae contain a range of chronic inflammatory cells, predominantly lymphocytes and macrophages, which vary in prevalence with the form of this spectral disease (12). Monoclonal antibodies (MAbs) have been used to examine the phenotype of these infiltrating cells within the tissue granulomas of leprosy patients. The characterization of T-lymphocyte subsets reveals consistent patterns of T-helper (CD4) lymphocytes and T-suppressor/cytotoxic (CD8) lymphocytes in multibacillary and paucibacillary leprosy. In the tuberculoid form of the disease, there is a mantle of CD8 cells surrounding the CD4-rich granulomas but lepromatous tissue lacks this organization and the relatively fewer CD4 and CD8 cells are scattered throughout the infected tissue (6,9,14).
The development of specific MAbs to M. leprae components, and those to related organisms, has provided an additional important approach to the investigation of leprosy through immunohistological and immunodiagnostic studies. For example, anti-BCG antibody has been used in combination with the peroxidase-antiperoxidasc technique to demonstrate mycobacterial antigens in conventionally fixed and paraffin-embedded skin and nerve biopsies of leprosy patients (78). Similarly, the MAbs ML04 and ML06-which recognize 35 kDa and 12 kDa proteins of M. leprae-detect antigens located in leprosy lesions and are expressed on the cells infiltrating these granulomas (10).
In the present report, a panel of MAbs (Table 1) directed against protein and carbohydrate components of M. leprae was assessed on biopsies from a range of clinical cases, and the localization and distribution of M. leprae antigens in these tissues are described.
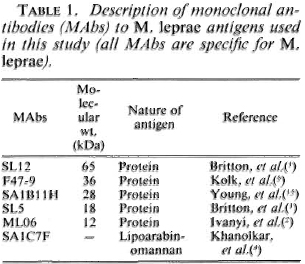
MATERIALS AND METHODS
Tissue specimens. Eighteen biopsies were taken from leprosy cases attending the clinic in the Central JALMA Institute for Leprosy, Taj Ganj, Agra, India, and classified histologically according to the criteria of Ridley and Jopling (13). The control tissues used in this study were surgically obtained skin samples from patients with onchocerciasis, leishmaniasis and individuals without dcrmatopathology. A portion of each biopsy specimen was fixed with Formalin/mercuric chloride/acetic acid (FMA) (12) and processed by paraffin embedding for histopathological study. The remainder was snap-frozen for immunohistological studies in Tissue Tek (Miles Laboratories, Inc., Elkhart, Indiana, U.S.A.) using isopentane. Table 1 gives the clinical and histopathological data for each of the tissues used. The methods used to determine the bacterial index (BI) in the tissues were those recommended by Ridley (12). The total content of the acid-fast bacilli (AFB) per gram of tissue was determined by homogenization of the samples (10 mg of tissue in 250 μl of phosphate buffered saline), and the BI was determined using a standard protocol (11).
Immunohistological staining. Detection of M. leprae antigenic sites present in the sections was achieved by using a two-step immunostaining with peroxidase (Sigma Chemical Co., St. Louis, Missouri, U.S.A.), fluorescein (Sigma), or alkaline phosphatase (Sigma) labeled reagents. Five-/mi cryostat sections were first incubated with the MAbs to M. leprae antigens as listed in Table 1. The MAbs were diluted 1:10 in Tris-HCl buffer with 5% normal goat serum and incubated for 1 hr in a moist chamber at room temperature (RT). After three washes in Tris-buffer at RT, the sections were incubated for 1 hr at RT with peroxidase or alkaline phosphatase labeled affinity purified goat anti-mouse immunoglobulin (IgG; Bio-Rad Laboratories, Richmond, California, U.S.A.) diluted 1:30 in Tris-HCl saline buffer with 5 % normal goat serum as the secondary reagent. Controls included the use of normal serum or omission of primary antibody or MAbs. Affinity purified goat anti-mouse immunoglobulin (IgG; Biorad) conjugated to peroxidase or alkaline phosphatase was used as the secondary reagent. Endogenous peroxidase was blocked with 0.228% periodic acid for 45 sec (3), and alkaline phosphatase was blocked with levamisolc. Peroxidase was developed using 3,3 diaminobenzidine (Sigma)/H202, and alkaline phosphatase with fast red. The sections were countcrstained with Mayer's hematoxylin. Peroxidase- or alkaline phosphatasestaincd tissue components were evaluated by two independent observers and scored semiquantitatively (i.e., - to + + +) for the amount of stain present.
RESULTS
Histopathological picture. The 18 cases were classified as shown in Table 2. The BI was determined in these biopsies by two different techniques. There was reasonably good concordance between the bacterial evaluation of the lesions by bacterial count and by histological assessment (Table 2). Both techniques were comparable except in two paucibacillary cases; the bacterial count failed to locate AFB in these two cases (Nos. 3 and 12) while histological assessment revealed the presence of one or two bacilli per section. A single acid-fast bacillus was seen in sections of three of the tuberculoid cases where tissue homogenization had revealed more.
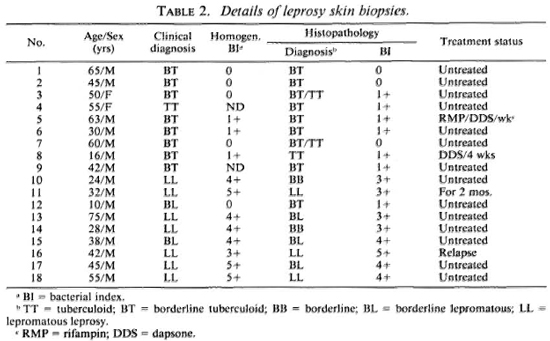
Immunostaincd sections. The distribution and intensity of positively stained areas varied with the different antibodies and markers used. There was also, as expected, variation in staining intensity between sections with different levels of AFB present. The multibacillary leprosy cases (i.e., BI > 3+) showed positive immunocytochemical staining using the peroxidase label with the majority of the MAbs used (Table 3). The staining was intracytoplasmic and granular in character (Figs. 1-6). The MAbs producing the strongest staining were against the 65 kDa and lipoarabinomannan (LAM) components. The immunostaining was not seen in the form of discrete whole or fragmented bacilli but appeared in a more diffuse state and was confined to cells that were morphologically indentifiable as macrophages. Paucibacillary leprosy sections, with a BI of 0 or 1+, showed less positive immunostaining or none at all (Table 3). In only one case did more than two MAbs stain the antigenic material in the macrophages. Two of the three biopsies in which no AFB were identified by either histopathological or tissue homogenization methods had weak but definite positive immunostaining with the MAbs against 36 kDa (two cases) and LAM (one case). The staining here was intracytoplasmic in the macrophages and had a finely granular appearance. No definite intraneural staining was identified.
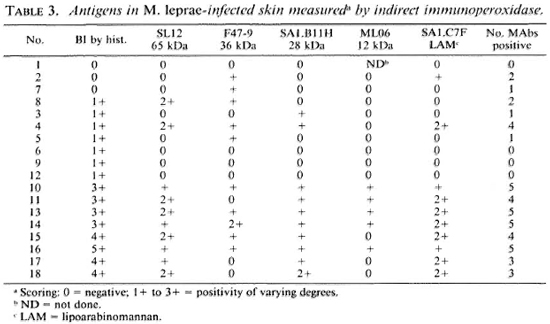
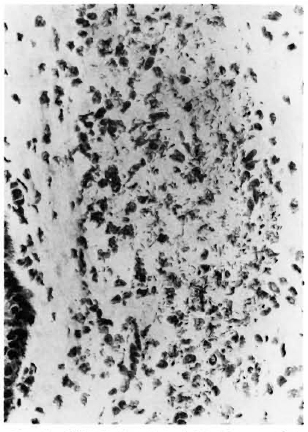
Fig. 1. Ziehl-Neelsen-stained skin-biopsy, section from a lepromatous patient showing many bacilli (x 1000).
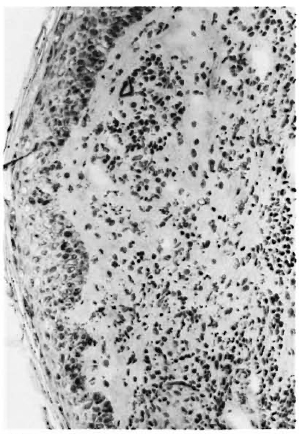
Fig. 2. Ziehl-Neelsen-stained skin-biopsy section from a paucibacillary patient showing no bacilli ( x 250).
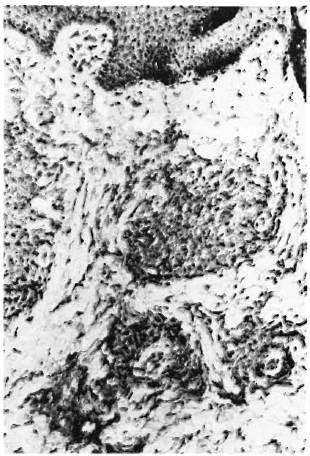
Fig. 3. Immunopcroxidase staining of a paucibacillary (BT) skin-biopsy section showing positive staining for M. leprae 28-kDa protein (x400).
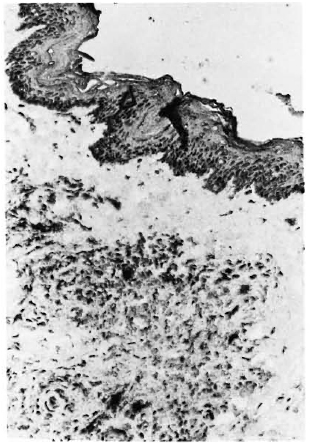
Fig. 4. Alkaline-phosphatase stain using MAb C7F for lipoarabinomannan in a sample of lepromatous skin ( x 400).
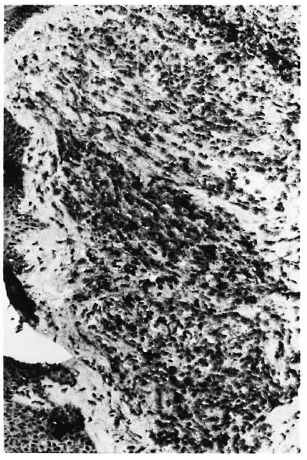
Fig. 5. Strongly positive immunoperoxidase staining of M. leprae 65-kDa protein in a skin-biopsy section from a patient with lepromatous leprosy (x250).
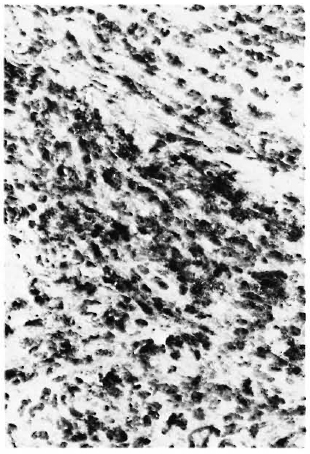
Fig. 6. High-power photomicrograph of tissue shown in Fig. 5 (x 1000).
Alkaline phosphatase was also tested as a marker reagent with most of the samples and with the different antibody combinations. However, this marker was not as consistent in revealing antigens as was the peroxidase reagent, even in the multibacillary cases. Control sections from patients with onchocerciasis and leishmaniasis showed no staining in the inflamed or normal dermis with any of the MAbs.
DISCUSSION
This study has shown that protein and carbohydrate antigens of M. leprae can be detected in histological sections of biopsies from patients with different forms of leprosy, including those apparently free of bacteria (BI = 0) when a reasonable number of Fite-stained sections are examined. The location of these antigens is confined to the areas of cellular infiltrate, and is most concentrated in cells with the morphology of macrophages. The patterns of immunostaining are consistent with previous findings in paucibacillary and multibacillary cases with nonspecific antimycobacterial antibodies such as anti-BCG or specific M. leprae MAbs to the 35 kDa and the 12 kDa components (7,10).
The histopathological diagnosis of leprosy conventionally utilizes modified Ziehl-Neelsen methods to identify AFB. In tissues that appear free of AFB, diagnosis may be difficult and inconclusive. In our study, of the 10 paucibacillary cases studied, 4 of the 7 which had AFB seen histologically also had positive immunostaining with MAbs against M. leprae. Conversely, 2 of the 3 in which no AFB were identified showed local positive immunostaining with one or two of the MAbs. In the study by Narayanan, et al. (10), all of the tuberculoid and lepromatous skin granulomas stained with two MAbs (ML04 and ML06, which recognize the 35 kDa and the 12 kDa antigens, respectively). However, the positive staining was on lymphocytes and macrophage membranes and the AFB did not stain. Our current observations are quite different, with positive AFB staining and cytoplasmic location correlating approximately with the AFB density by Fite-Faraco estimation. The present findings are more comparable with the study of neural tuberculoid leprosy reported by Mshana, et al. (8) in which AFB were visualized in 6 of 12 biopsies from borderline tuberculoid (I3T) patients, while 11 of 12 had demonstrable antigen in their nerves using anti-BCG polyclonal antibody. In later cases, the detcctability of antigen in nerves dropped to zero, but at a slower rate than detcctability of AFB.
The location of staining, and therefore antigens, in macrophages is to be expected. However, since these staining patterns are not in the forms of bacilli it is probable that the antigens being identified by these MAbs are breakdown products, or have been released from bacteria. The cellular location of these antigens and their relationships to bacilli in organism-positive tissues require further study at the ultrastructural level.
It has been suggested that the immunological defect in leprosy is reflected in the degree of bacterial (and therefore antigen) load present. Thus, it will be important to use an immunocytochcmical approach to readdress this question and to determine more specifically the nature of the antigens, their distribution, and their concentration in the different states of leprosy. The ability to detect various antigenic fractions in tissues will provide information on their persistence, their clearance, and which cells are involved in the handling of these antigens. The differences in positivity between sections from different patients of the same bacterial grade may reflect a variation in tissue distribution of the antigens identified by the particular reagent. It is not unreasonable to suggest that there could be different amounts of bacterial antigens present in different samples.
Our present results using MAbs in frozen sections will be helpful in identifying M. leprae components in tissues when AFB are not detectable by standard histological means. However, the technique remains relatively complicated and requires fresh tissue and frozen-section techniques. Although its applicability for diagnostic purposes is perhaps limited, it may nevertheless be a useful immunopathological tool for studying cases when AFB are not identifiable by standard histopathology.
Acknowledgments. This work was supported by the THELEP component of the UNDP/World liank/WHO Special Programme for Research and Training in Tropical Diseases and from the Wolfson Foundation (London). We thank Drs. W. J. Britton, J. Ivanyi, and A. H. J. Kolk for kindly providing the monoclonal antibodies for this study, and Dr. R. J. W. Rces for arranging for the biopsies.
REFERENCES
1. BRITTON, W. J., HELLQVIST, L., BASTEN, A. and RAISON, R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
2. IVANYI, J., SINHA, S., ASTON, R., CUSSEL, D., KEEN, M. and SENGUPTA. U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin. Exp. Immunol. 52(1983)528-536.
3. KELLY, J., WHELAN, C. A., WEIR, D. G. and FEIGHERY, C. Removal of endogenous peroxidase activity from cryostat sections for immunopcroxidase visualisation of monoclonal antibodies. J. Immunol. Methods 96(1987)127-132.
4. KHANOLKAR, S. R., YOUNG, D. B., BRENNAN, P. J., BUCHANAN, T. M. and MCADAM, K. P. W. J. Use of an antigen-capture assay for characterisation of monoclonal antibodies to mycobacterial lipoarabinomannan. J. Med. Microbiol. 28(1989)157-162.
5. KOLK, A. H. J., Ho, M. L., KLATSER, P. R., EGGELTE, T. A., KUIJPER, S., DE JONGE, S. and VAN LEEUWEN, J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and .1/. leprae. Clin. Exp. Immunol. 58(1984)511-521.
6. MODLIN, R. L., HOFMAN, F. M., MEYER, P. R., SHARMA, O. P., TAYLOR, C. R. and REA, T. H. In situ demonstration of T lymphocyte subsets in granulomatous inflammation: leprosy, rhinosclcroma and sarcoidosis. Clin. Exp. Immunol. 51(1983)430-438.
7. MSHANA, R. N., BELEHU, A., STONER, G. L., HARDOE, M. and HAREGEWOIN, A. Demonstration of mycobacterial antigens in lepromatous tissues. Int. J. Lepr. 50(1982)1-10.
8. MSHANA, R. N., HUMBER, D. P., HARBOE, M. and BELEHU, A. Demonstration of mycobacterial antigens in nerve biopsies from leprosy patients using peroxidasc-antipcroxidasc immunoenzyme technique. Clin. Immunol. Immunopathol. 29(1983)359-368.
9. NARAYANAN, R. B., LAAL, S., SHARMA, A. K., BHUTANI, L. K., and NATH. I. Differences in predominant T cell phenotypes and distribution patterns in rcactional lesions of tuberculoid and lepromatous leprosy. Clin. Exp. Immunol. 55(1984)623-628.
10. NARAYANAN, R. B., RAMU, G., SINHA, S., SENGUPTA, U., MALAVIA, G. N. and DESIKAN, K. V. Demonstration of Mycobacterium leprae specific antigens in leprosy lesions using monoclonal antibodies. Indian J. Lepr. 57(1985)258-264.
11. REES, R. J. W. Limited multiplication of acid-fast bacilli in the foot pads of mice inoculated with Mycobacterium leprae. Br. J. Exp. Pathol. 45(1964)207-218.
12. RIDLEY, D. S. Skin Biopsy in Leprosy. 2nd ed. Basle: CIBA-GEIGY, Ltd., 1985., p. 1.
13. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
14. VAN VOORHIS. W. C, KAPLAN, G., SARNO, E. N., HORWITZ, M. A., STEINMAN, R. M., LEVIS, W. R., NOGUEIRA, N., HAIR, L. S., GATTASS, C. R., ARRICK, B. A. and COHN, Z. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N. Engl. J. Med. 307(1982)1593-1597.
15. YOUNG, D. B., FOHN, M. J., KHANOLKAR, S. R. and BUCHANAN, T. M. Monoclonal antibodies to a 28,000 mol. wt protein antigen of Mycobacterium leprae. Clin. Exp. Immunol. 60(1985)546-552.
1. Ph.D., Research Fellow; Wolfson Tropical Pathological Unit, London School of Hygiene and Tropical Medicine. Keppel Street. London WC1E 7HT, U.K.
2. M.R.C.P., M.R.C.Path., Senior Lecturer; Wolfson Tropical Pathological Unit, London School of Hygiene and Tropical Medicine. Keppel Street. London WC1E 7HT, U.K.
3. M.D., M.Sc, Student; Wolfson Tropical Pathological Unit, London School of Hygiene and Tropical Medicine. Keppel Street. London WC1E 7HT, U.K.
4. F.R.C.P., Head, Department of Clinical Sciences; Wolfson Tropical Pathological Unit, London School of Hygiene and Tropical Medicine. Keppel Street. London WC1E 7HT, U.K.
5. M.R.C.Path., Ph.D., Senior Lecturer, Wolfson Tropical Pathological Unit, London School of Hygiene and Tropical Medicine. Keppel Street. London WC1E 7HT, U.K.
6. M.D., Assistant Director; Central JALMA Institute for Leprosy. Taj Ganj, Agra, India.
7. M.D., Senior Research Officer, Central JALMA Institute for Leprosy. Taj Ganj, Agra, India.
Received for publication on 27 January 1989.
Accepted for publication on 24 April 1989.