- Volume 57 , Number 3
- Page: 671–86
Immunological tools in leprosy control*
Editorial opinions expressed are those of the writers.
XIII LEPROSY CONGRESS STATE-OF-THE-ART LECTURE
We are pleased to have the opportunity of publishing the full texts of the state-of-the-art lectures presented at the XIII International Leprosy Congress at The Hague, The Netherlands, 11-17 September 1988.
Recent years have witnessed a remarkable burst of immunological activity in the leprosy literature. More publications are now appearing on the immunology of leprosy than on any other aspect of the disease. More sessions at this Congress arc devoted to immunology than to any other aspect of leprosy. The dominance of this research - or at least these publications - is itself an interesting phenomenon. It represents what is, in effect, a delayed immunological response to the armadillo - in the sense that the discovery in 1970 that the armadillo could support Mycobacterium leprae meant, for the first time, the potential availability of antigen for basic research, but this had first to be made an actual availability before it could attract funding agencies and then basic scientists into the field of leprosy. The most obvious example of this activity has been the IMMLEP program of the World Health Organization (WHO), a program which has spent approximately half its budget since 1974 on rearing armadillos and which has provided funding and M. leprae antigens for many immunological projects around the world. Our current literature carries the results of the consequent influx of scientific energy.
This burgeoning immunological literature describes a wide variety of experiments and observations relevant to leprosy. If we read the discussion sections of the many papers we are generally told that the research has actual or potential implications for ultimate control of the disease. Even if only a small proportion of these potential leads is ultimately successful in producing practicable tools, the very size of this literature should itself be an encouraging sign for those actually involved in leprosy control, and for those suffering from or at risk of the disease.
But there is another view which says that enthusiastic research and publications and promises are all very well, but which would argue that the reality of leprosy control in the world today has not been affected at all by these recent immunological developments. Some might even express scepticism that immunological tools, as distinct from drugs and health education and hard work in the villages, will ever contribute much to leprosy control. I suspect that many views on this issue are represented here at this Congress, and that many of the papers which are to be presented over the next several days reflect, implicity or explicity, these divergent opinions.
I have been asked to examine this question, of the actual and potential contributions of immunological tools to leprosy control. It is a formidable challenge, given the complexity of the subject, and the conflicting views-sometimes extravagant claims-made about it. It is also an important challenge, given that we have here, at this Congress, one of the largest gatherings ever of individuals who work on the basic science and basic immunology of leprosy, and also of those individuals who are directly involved in the day-to-day demands of controlling the disease in all parts of the world. We have here an important opportunity to learn from one another. I would thus hope that this session can begin a process of exchange and communication between the many groups of experts represented at this Congress, to facilitate progress toward what is our common goal: reduction of the morbidity associated with leprosy around the world.
There are at least two ways of approaching the subject of immunological tools for leprosy control. One would be to review the variety of immunological phenomena reported in the literature, and to predict or imagine how each might be applied in controlling the disease. Another is to consider the demands of leprosy control, and then to scan the recent literature for signs of helpful tools or observations. I have decided upon the latter approach for this review insofar as it provides a logical structure against which to view current immunological research relating to leprosy. More importantly, it provides an opportunity to emphasize the reality and demands of leprosy control in today's world as a set of constraints against which to measure the practicability of available and potential immunological tools. It is a question of jobs which are in need of tools, not of tools in need of jobs.
Leprosy control
In basic textbooks, disease control is often described as consisting of three sorts, or levels, of activity: primary (prevention), secondary (treatment), and tertiary (rehabilitation). In actuality, leprosy control has for the past 30 years been conceived almost entirely in terms of secondary interventions, that is, the finding and treatment of leprosy cases. It should be appreciated that this work has been carried out, and must continue to be carried out, under extremely difficult conditions, more often than not in rural areas of poor countries. Problems of difficult access, of ignorance and stigma, and of the absence of amenities, such as electricity mains, are all very much part of the reality of leprosy control. Serious though the disease may be, leprosy is nowhere among the top priorities in health and thus a shortage of funds, and of logistic and supervisory support, are common to most control programs. That is the situation to be worked with.
Despite these difficulties, leprosy control is today carried out in most leprosy-endemic populations of the world. For the purposes of this review, we may consider these leprosy control activities as involving several steps: case prediction, case finding, case diagnosis, case management, case surveillance, case rehabilitation and, of course, the ultimate goal, case prevention. That is what leprosy control means. Now let us examine what immunological tools are or might soon be available for each of these tasks.
Case prediction
It is, or would be, useful to be able to predict who is going to develop clinical leprosy so as to be able to target prophylaxis or case finding. Indeed, the prediction of disease-in the sense of the identification of high-risk individuals or groups-is already an important part of many routine leprosy control programs, at least insofar as household contact tracing is carried out. This exercise is based upon evidence that household contacts have predictably high risks of developing leprosy, as has been demonstrated in many epidemiological studies. 12 The rationale has thus been that as we recognize household contacts to be at particularly high risk of developing disease, it is cost-effective in terms of case finding activity, and perhaps even necessary in terms of ethical responsibility, to examine them repeatedly so as to be able to identify and treat any new disease at the earliest possible stage.
Although the elevated risk among household contacts has been amply demonstrated, its explanation is still unclear. It has often been interpreted as reflecting shared genetical susceptibility within families, though this explanation has not been formally confirmed. Closeness of genetical relationship is typically confounded with closeness of physical contact, and the only study which has attempted to separate the two influences found that proximity of living contact explained the elevated risk as well as did proximity of genetical relationship.3 Elegant immunogenetical studies of the past ten years, mainly by Dr. R. R. P. de Vrics and his colleagues in this our host country, have revealed strong evidence for HLA-linked genetic control for some determinants of leprosy type, but not for leprosy risk.4-5 Evidence for genetical factors associated with leprosy per sc is much less strong, and it is in this sense that the increased risk of leprosy among household contacts of cases is still unexplained.
Case prediction in household contacts might, in theory, be made more efficient if we had sensitive and specific tools with which to identify the individuals actually destined to develop leprosy. On the other hand, it is widely appreciated that although household contacts have high relative risks of developing the disease, the majority of cases in endemic communities arise among individuals without obvious household contact relationship with known cases (Table 1). 6 This poses a major dilemma for any approach to case prediction, insofar as it means that large populations in endemic areas would have to be screened for predictive factors if a high proportion of all cases were to be identified before onset. Given that an incidence rate of 1 case of leprosy per 1000 population per year is high, it means that thousands of screening tests must be carried out to identify only a few presumptive cases.
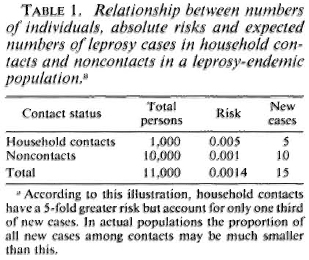
One may question the cost benefit and a cost effectiveness of such an exercise. The only way to reduce the cost per case predicted is to first narrow down the population at risk by some simple screening criterion before applying the more sensitive and specific test. But this is just the rationale for focusing on household contacts, and is what led to the problem of missing the forest for the trees-of missing the majority of incident cases. It is a major practical problem, and one to which we will later return.
However, for the purpose of argument, let us suppose that we surmount any barriers of cost and logistics and are able to carry out very large numbers of screening tests in an endemic population with the intent of identifying those who are destined to develop clinical leprosy. What tests would we apply? We have three sorts to choose from: immunogenetic tests, skin tests, and serological tests. Recent years have witnessed interest in all three.
Immunogenetic tests.
The primary rationale for immunogenctical studies on leprosy has not been that their findings might be applied directly in the context of control but, rather, that they may give some clues to the molecular and cellular mechanisms underlying the response to infection with M. leprae. This research has been eminently successful in identifying the importance of HLA-D antigens which are involved in mediating reactions between macrophages and T-hclpcr or -suppressor cells and which exert some influence over whether an individual case will manifest tuberculoid or lepromatous disease. The frontier of genetical work in leprosy has thus moved away from human population and family studies to in vitro studies of genetically defined cell lines.7 Perhaps this will someday lead to an understanding of why some individuals develop multibacillary as distinct from paucibacillary disease. Let us hope so.
In the meanwhile, there are exciting times ahead for the population genetics of leprosy as researchers begin to take advantage of the fact that the human genome has now been almost completely mapped, giving us polymorphic chunks called restriction fragment length polymorphisms (RFLPs) on all the chromosomes, whose distribution within families can be compared with leprosy status. No doubt new genetical associations will emerge in the next few years. But it is hard -at least for me -to conceive of the routine screening of populations to identify high-risk individuals on the basis of genetical markers, certainly not in the foreseeable future. Among the opposing arguments would be ethics, low specificity, and cost. Ethics, insofar as genetic markers for leprosy susceptibility would be stigmatizing. Low specificity, in that leprosy is, after all, an infectious disease, and many people may have a genotype for susceptibility but never contract leprosy, if only because they never meet the leprosy bacillus. And cost -well, I wish there were but there just isn't that kind of money available for leprosy in today's world. It's out of the question. So let us look at the other options.
Skin tests.
Skin and antibody tests have greater theoretical potential for case prediction than do genetical tests, insofar as they might be able to identify actively infected individuals as well as to reflect their immune response to that infection. In this sense, we may speak of "early detection" of cases as the goal. Skin tests have a particular appeal, given that they are so simple to perform with no requirement for specimen collection or laboratory backup. These advantages, together with the fact that tuberculin is a useful tool in identifying individuals at high risk of developing tuberculosis, led to work on analogous skin-test preparations using M. leprae antigens. Several such antigens-now generally called M. leprae soluble antigens (MLSAs)-have been prepared, by different protocols, mainly by Dr. J. Convit in Venezuela and by Dr. R. J. W. Rees in London, and we are now seeing published results of studies with these reagents in different populations.8-13 There appear to be considerable differences between populations in their responses to these skin tests but, in general, the proportion positive increases with age, until about 30 years of age, and is higher in BCG-vaccinated than unvaccinated individuals. These patterns are illustrated in Figure 1, showing data based on one of the Rees-typc antigens as applied in the Lepra Evaluation Project in northern Malawi.12,14
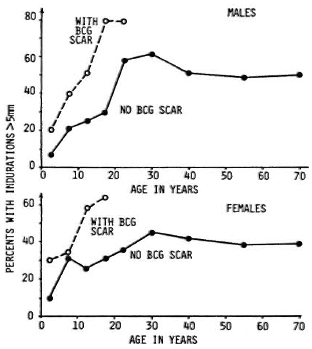
Fig. 1. Distribution of sensitivity to Rees-type M. leprae soluble antigen (batch AB) by sex, age, and BCG scar status in Karonga District, northern Malawi.12,14 "Positivity" defined as an induration > 5 mm at 48 to 72 hr.
Virtually all of the MLSA studies thus far have been cross sectional, thus we have yet to sec what are the long-term implications of positivity or negativity to such tests. On the other hand, it is obvious from Figure 1 that the predictive value-the proportion of positive (or negative) tests which actually reflect imminent disease -will be very low. The interpretation of a positive skin test is problematic insofar as several studies have shown that none of the current MLSA reagents is specific for M. leprae exposure or infection. BCG vaccination induces sensitivity to them, and it is presumed that a number of other mycobacterial infections do so as well.12 And to make things worse, the information to date indicates that the antigens are only approximately 50% sensitive even in picking up paucibacillary cases, although these cases should, in theory, have strong cell-mediated immune responses to antigens of M. leprae.13 The interpretation of a negative skin test raises a different problem. It may be that a very high proportion -even all -of future multibacillary cases arise from the group which is negative to some skin test, but this group will also include a proportion of future paucibacillary cases as well as large numbers of individuals who are skin-test negative because they have never met M. leprae or any other sensitizing antigen. Given these problems, we must conclude that current soluble skin-test antigens have no operational usefulness at all in the context of leprosy control. It may be that careful analysis of epidemiological data based on skin testing with these antigens will be useful in revealing important interactions between sensitizing mycobacterial antigens. Only time will tell.
Serological tests.
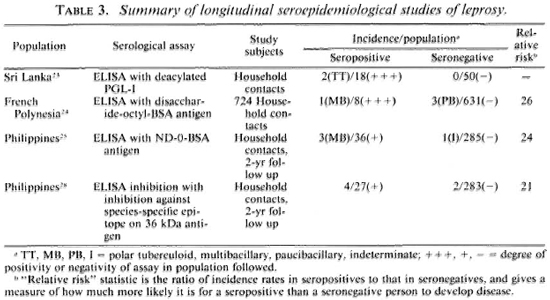
This problem of test interpretation may be less severe in the case of serological assays, at least in theory, insofar as a strong humoral antibody response might reflect both infection with M. leprae and a destiny to develop multibacillary disease. Thus, there has been much interest in serological methods, beginning with the fluorescence approach (FLA-ABS test) of Abe and shifting, in recent years, to ELISA methods using in particular the natural phenolic glycolipid (PGL-I) antigen of M. leprae or its synthetic analogues (e.g., the terminal disaccharide linked to bovine serum albumin), or to assays based upon monoclonal antibodies specific to M. leprae antigens.15-1 6 The tests which are currently of greatest interest and application are summarized in Table 2. One of the difficulties in reviewing this literature is the fact that many different assays and assay protocols have been employed. Only the ELISA test based upon PGL-I antigens has been used widely and compared between laboratories. 16,19,20 Another difficulty is that most of the seroepidemiological studies reported thus far have been cross sectional, and very little follow-up information is yet available.
The cross-sectional studies have shown that we now have several serological tools which can identify specific antibodies to M. leprae consistently in bactcriologically positive multibacillary patients. The results in paucibacillary patients are far less promising however and, in general, it is found that it is not possible to "detect" even half such cases, with any of the assays, without a drastic reduction in test specificity.21,22,28 This problem is illustrated in Figure 2, which shows the proportions of multibacillary cases, paucibacillary cases and nonleprosy controls identified using a standard ELISA test based on synthetic PGL antigen, with different criteria for test positivity.
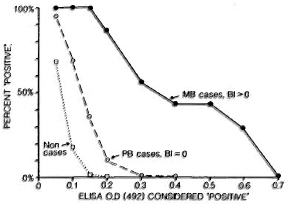
Fig. 2. Proportions of untreated multibacillary and paucibacillary cases considered "positive" by an ELISA test using synthetic disaccharidc-BSA antigen with different criteria for positively. Also shown are proportions of nonleprosy controls who would falsely be declared "positive" at each criterion (extracted from 21).
Table 3 summarizes the published results of follow-up studies showing the implications of serological status for predicting subsequent disease. We see evidence that the antibodies are indeed predictive, at least in a statistical sense, but the results also raise several major practical issues. The first is the issue of deciding what it is we wish to predict. The tests appear to be best at predicting multibacillary disease. This may not be surprising, given what we know of immune responses across the leprosy spectrum. It is sometimes argued that the early detection of multibacillary disease is most important because such cases are major sources of transmission of M. leprae in endemic populations.2 But two other points might usefully be introduced into this debate over the rationale for early detection by serology. First, although the epidemiological evidence that household contact with a multibacillary case is an important risk factor still holds, the recent literature includes increasing evidence for other sources of M. leprae transmission, for instance, contact with armadillos in Mexico.32,33 I do not wish to push this argument too far, since the evidence for multibacillary cases as important sources still stands, but nor do I wish to neglect what I detect as a shift in the recent literature away from a view that multibacillary cases are the sole sources of infection in endemic communities. Secondly, it should be appreciated that multibacillary cases are by no means the only ones of clinical importance, insofar as the majority of leprosy cases, and in many parts of the world the majority of leprosy morbidity, is attributable to paucibacillary disease. So this, too, may affect the argument and rationale for investing heavily in the early detection of multibacillary disease alone.
The interest in predicting multibacillary disease highlights another problem, that of numbers. Note that all of the data cited in Table 3 relate to household contacts, a well-recognized high-risk group for clinical disease. However, as noted above, many investigators have commented that only a minority of all incident cases in endemic communities arise in this group. Furthermore, the incidence rate of multibacillary disease in general populations is typically so low it exceeds 1 per 10,000 per year in very few populations-that a very large expenditure would be required to identify very few cases. The magnitude of this task must be appreciated. Quite apart from collection and testing of specimens-and note that specimen collection would have to be repeated periodically-this implies a very large data-processing task to keep track of thousands of specimens and to ensure that source individuals can be identified in the future. This means large numbers of forms, registers, clerks and supervisors, and the inevitable bothers which arise when a complicated data operation goes wrong.
There is another side to this numerical problem. Although Table 3 shows a clear statistical association between serological positivity and leprosy risk, we note that the majority of test positives did not-at least in the time frame of these studies-develop disease. Thus a large majority of the positive results appear to have been "false." This proportion of false-positive results would have been even higher-thus the predictive value of the assay would have been even lower-if the assay had been applied to a noncontact group with lower overall risk of developing disease. This is a necessary implication of the rarity of leprosy on the one hand, and of imperfect test specificity on the other. This relationship is illustrated in Table 4. We see for example that even if a test is 99% specific-and none of our tests is yet that good-and the true risk of leprosy is one per thousand, then 10 out of 11 (90.9%) of our test positives will be false. One may question whether such errors can be tolerated on ethical, logistic or economic grounds within the context of a real leprosy control program.
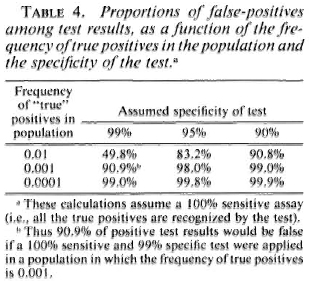
Taken together, such arguments weigh heavily against the practicability of extensive serological screening for large-scale leprosy control programs. The cost effectiveness equation should be most favorable in small communities with high disease prevalence and incidence and ready access to sophisticated laboratory services, but even under these conditions, it has yet to be demonstrated that serological screening is or could be worthwhile.
Case finding and case diagnosis
The finding and diagnosis of leprosy cases rely largely upon clinical signs, supplemented in many control programs by slit-skin smear bacteriology. Only in exceptional situations such as nonendemic countries and research projects are biopsies involved routinely in the diagnostic procedure. We may ask whether any available immunological tool is able to contribute to the process.
Current skin tests are of low sensitivity and low specificity for paucibacillary disease and of low specificity for multibacillary disease. Serological assays-ELISA based on PGL-I or its related antigens, or monoclonal antibody competition assays-have low sensitivity for paucibacillary disease, which presents the greatest diagnostic difficulty. On the other hand, one might argue that serology could be of use in diagnosing, confirming or classifying multibacillary disease, given its high sensitivity in this context. This raises the question of whether a serological test is or can be more practical than traditional slit-skin smear bacteriology.
Several studies have now recorded a significant correlation between bacteriological status as measured by slit-skin smear bacterial index (131) and antibody titers, in particular antibodies to PGL-I.19,34 Figure 3 shows an example of such data based on the same ELISA protocol and performed by two laboratory groups. Two comments are in order about this figure. First, if we look at these data closely, we sec that the correlation coefficient is a function of the range of BI values associated with the test sera. Second, we note that the correlation, although highly significant statistically, includes a considerable degree of scatter. It should be appreciated that this scatter is not necessarily a reflection of the ELISA method - indeed, it is likely that there is greater observational error in the BI variable.
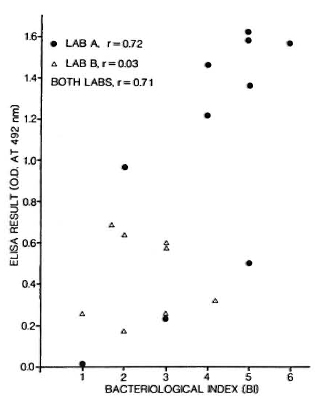
Fig. 3. Relationship between ELISA result and bacterial index (BI) in 18 bactcriologically positive leprosy patients; 10 were tested in one laboratory (A), 8 in another (B) using same synthetic dissacharide-BSA conjugate antigens.
Given that slit-skin smear examination is now routine in many leprosy control projects, we may ask whether this could be replaced by routine serological testing. On first examination the answer might seem to be no, given that the taking and reading of slit-skin smears is in principle a simpler task than is the collection of blood or serum and the performance of an ELISA test. On the other hand, the routine slit-skin smear practice leaves much to be desired. A recent review of routine slit-skin smear services in leprosy control projects found the results so unreliable that the authors questioned whether the services were of any use at all.3 5 The reading of slit-skin smears is an extremely tedious task and inevitably prone to considerable human error. In contrast, ELISA tests can be carried out in large numbers-in a central or reference laboratory - and can be automated to provide objective results. In addition, ELISA tests can be performed on sera eluted from a few drops of blood collected by finger prick and dried on absorbent paper, a procedure which is even simpler than the collection and preparation of slit-skin smears.36,37
We may thus have arrived at an interesting juncture, since the acceptance of slit-skin smear services as a routine part of a local leprosy control program may at least be questioned. ELISA testing on capillary blood may now provide a more reliable and even cheaper alternative, because of its standardized procedures, objective result, and the economics of scale associated with a central laboratory. A major question in this equation relates to time delays and the method of shipment of blood or serum from the field to a central laboratory. If a cold chain is required, then this will reduce its practicability. On the other hand, if adequate results can be obtained from blood spots dried on filter paper and shipped without thermal control, then the balance may now favor the institution of centralized ELISA testing in place of regional slit-skin smear services, at least in some areas with reasonable access to a laboratory. Unfortunately, the current PGL-I ELISA tests depend upon IgM antibodies, which are less stable than IgG, and may thus not travel well in a dried state or without thermal control. Just how important this may be has yet to be assessed, but the approach is at least worth a serious operational trial.
Case management
Routine treatment of clinical leprosy is largely a matter of the provision of proper antimicrobial drugs. Multiple drug therapy (MDT) is now widely practiced throughout the world with different regimens recommended for paucibacillary and multibacillary patients. Although these two patient groups are conventionally defined in terms of 131 assessed through a slit-skin smear,3 8 there may be a role for an immunological test in this context. The lepromin test is, or at least has been, used for this purpose in some areas, particularly in South America, but I am not aware of any rigorous studies comparing its usefulness against a bacteriological criterion. More reasonable may be the use of a serological assay and criterion, as discussed above in the context of case diagnosis.
Several recent studies have shown a decline in antibody titers with the course of treatment of multibacillary patients, correlating with the fall in BI, and it has been suggested that serological assays might be used to monitor therapy and to determine the time when treatment can be discontinued. 39,41
Once again, there is a need to carry out a rigorous comparison of the relative advantages and disadvantages of a centralized serological testing versus regional slit-skin smear service. Such a comparison will be complicated by the fact that leprosy-control thinking has for decades been based upon slit-skin smear information, and there will be reluctance to shift unless the serological tests can be shown to be unambiguously superior.
Also important in case management are a variety of drugs used to manipulate the immune response in leprosy cases, in particular immunosuppressives used in treating reactions. Prednisolone and other corticosteroids are standard in the treatment of type 1 reactions and various antiinflammatory agents are used routinely in type 2 reactions.42 These chemical manipulators of the immune response may not be what one usually thinks of these days as immunological tools, but they are just that and they are extremely important in the management of leprosy disease. The importance of treatment of such reactions, and of the less dramatic but equally important so-called "silent" neuritis of leprosy, deserves some emphasis. These immunopathological processes are of extreme importance in leprosy control since they are responsible for the nerve damage and, hence, morbidity of the disease. There has been relatively little attention to basic research on these issues to date, despite encouragement by various organizations-most recently by Drs. Engers and Ji of the WHO IMMLEP program 43 - to stimulate such research.
Less controversially characterized as an immunological tool, but more controversial in usefulness, are different immunotherapcutic interventions based upon injection of antigens to stimulate or modulate a host's immune response. The literature records several early attempts to stimulate cell-mediated responses in CMI-deficient multibacillary cases by repeated injections of lepromin or BCG.44,45 None of these studies was carried out in a properly controlled fashion, however, and the results were never convincing enough for such approaches to be widely accepted and used. Recent years have witnessed a revival of interest in this approach, however, beginning with Convit's experience using repeated injections of mixtures of BCG plus killed M. leprae in the treatment of Mitsuda-negative leprosy patients in Venezuela. The results have been sufficiently promising for Venezuelan leprologists to have used the procedure on more than 1000 patients in the past 15 years.46 More recently, similar studies have been taken up in India by Dr. Deo and colleagues and by Dr. Talwar and his colleagues, who have used killed ICRC bacilli and killed Mycobacterium w., respectively, in BL and LL patients.47-48 Their preliminary results indicate immunological and histopathological changes similar to those reported by Dr. Convit. Despite enthusiastic reports of such interventions by some groups carrying out these studies, the methods are not widely accepted as useful tools in the treatment of leprosy cases. This scepticism is, in turn, a reflection of the fact that vaccine immunotherapy has not yet been convincingly demonstrated to be of value in a proper controlled trial. Several trials of BCG plus killed M. leprae immunotherapeutic vaccine regimens have recently been organized in The Philippines, China, and France, with support from the WHO THELEP group. On the other hand, these initial trials are being carried out in smear-negative ex-multibacillary patients; thus it will still be some years at least before we have convincing data one way or another concerning any value of such treatment regimens in the management of clinically active multibacillary leprosy.
Also in this context of immunotherapy, there should be mention of the current interest in the possible use of certain chemical mediators of immune reactions in order to reverse or replace specific deficits in the cellular immune response machinery of multibacillary patients. The most important such mediators thus far studied are interleukin-2 (IL-2)49-5 0 which is involved in communication between lymphocytes, and gamma-interfcron,51-5 2 which is involved in macrophage activation. There is evidence that at least some lepromatous patients may be deficient in these mediators, both of which are now available as pure polypeptides through recombinant DNA technology. Investigations involving the addition of IL-2 in vitro to lymphocytes from lepromatous patients have shown inconsistent evidence for correction of a prior immune deficit.49,50 And the injection of low doses of gamma-interferon into lepromatous skin lesions has shown some evidence for localized histopathological upgrading.51,52 This work is interesting but still in its early stages, not yet ready for clinical trials, let alone incorporation into the standard armamentarium of leprosy control.
Case surveillance
The recent shift to short-course drug regimens has introduced a new problem into leprosy control -that of surveillance during the months or years after completion of the prescribed therapy. The risk of relapses during this post-treatment period is not known, but of universal concern. Current recommendations, for example, by the WHO, are that ex-paucibacillary patients should be examined annually for 2 years and ex-multibacillary patients should have slit-skin smears performed yearly for a minimum of 5 years after completion of therapy.5 3 It is possible that serological surveillance of these patients could provide a more sensitive indicator of bacteriological relapse than can repeated slit-skin smear examinations. This is suggested by the recent evidence of declining antibody levels during the course of chemotherapy, paralleling declining BI,39-4 1 and by investigations of armadillos showing that anti-PGL antibodies are detectable in serum before bacilli can easily be detected in the skin.5 4 On the other hand, the recommendations for surveillance may themselves be changed in the next few years. They were based upon a philosophy of caution in the early years of MDT treatment. If it is found that the incidence of relapses is very low, then the rationale for expending much effort on post-treatment surveillance will itself be called into question.
Case rehabilitation
This is an important aspect of leprosy control, though one which is sometimes forgotten in basic research circles. Disabled patients require assistance of many kinds, from reconstructive surgery, physiotherapy and ulcer care to economic, social and psychological support. Unfortunately, this seems to be one area of leprosy control where immunological tools have little to offer. But it requires mention in that it is important, and will compete for the funds available for leprosy control.
Case prevention
We come at last to case prevention -"primary prevention" according to conventional terminology -the most attractive approach to disease control. Prophylactic vaccination is of course the example par excellence of an immunological tool for disease control. It is probably in this context that the most hopes have been pinned upon immunology and immunologists as potential saviors in the battle against leprosy. The possibility of such vaccines has received wide coverage in both the scientific and lay press in recent years. It is unfortunate that much of this coverage has been misplaced in its optimism, neglecting the obvious for the sake of less likely alternatives.
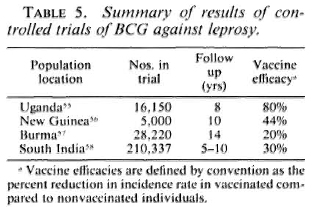
The story of vaccination against leprosy is inextricably intertwined with the story of BCG - from early arguments that tuberculosis and leprosy were antagonistic diseases to the demonstration by Fernandez and many others that BCG vaccination can elicit Mitsuda sensitivity, and on to the organization of four major controlled trials of BCG against leprosy, as carried out in Uganda, Burma, New Guinea, and South India.55-59 The results of these trials are well known (Table 5). BCG imparted statistically significant but variable protection against leprosy in each of the populations studied. The reasons for the variations are still unknown, but the message is clear-different BCG vaccines have been observed to impart significant protection against leprosy wherever they have been tested. This protection has carried to multibacillary as well as to tuberculoid disease wherever there were sufficient numbers of cases for the comparison to be made.59
Against this background should be set the fact of the widespread use of BCG throughout the leprosy-endemic world over the past three decades. Indeed, after the cessation of smallpox vaccination in the late 1970s, BCG became the most widely used vaccination in the world until the mid-1980s, when it was overtaken by polio vaccines. More than 2 x 109 doses have been given, and more than 50% of the infants in developing countries have received BCG in the past several years.6" But it is not just infants-BCG was introduced into many Third World countries 20 years ago in mass campaigns which achieved high coverage among children up to 15 years old. The effects of these campaigns are illustrated in Figure 4, showing data from a rural district in northern Malawi, central Africa. This shows that, in the early 1980s, approximately 60% of the population below 30 years of age had scar evidence of having received BCG vaccination. Similar patterns of BCG coverage are to be seen in most populations in the leprosy-endemic world. If we put these two facts together, the percentage of the population vaccinated and the protective efficacy, we predict overall effects on pre-BCG vaccination incidence rates as illustrated in Figure 5. Ofcourse, this simple analysis should not be pushed too far. Coverage rates are variable within and between populations; highest coverage may not always coincide with highest potential risk, and cohort effects-the fact that BCG was only distributed widely within the past 30 years -mean that relatively few adults in many endemic countries have been vaccinated. Nevertheless, I have little doubt but that the extensive use of BCG in the world is having an appreciable impact on the global incidence of leprosy. Recent reports by Lcchat and colleagues 62 and by Ponnighaus and Boerrigter 63 have shown evidence for falling incidence rates of leprosy in many populations-including China, Japan, Polambakkam in India, Venezuela, Burkina Faso and Malawi -and Noordcen and Lopez Bravo have noted a fall in leprosy case numbers in Africa over the past 20 years.64 Most of these declines antedated the mass use of BCG, and they are based upon routine data which are notoriously difficult to interpret. Nevertheless, I suspect that BCG is at least contributing to these declines. This docs not mean that leprosy is about to disappear. It isn't. This does not mean that BCG is an optimal vaccine. It clearly is not. But it does mean that when considering current and future trends of leprosy in the world we should not neglect the huge cohort of BCG-vaccinated individuals -literally numbering in the billions-who are assuming the majority of the world's population. In some populations BCG may have tipped the balance against perpetuation of the leprosy bacillus and, thus, be itself responsible for the continued downward trend in disease incidence. BCG is a fact, a very complicated fact, a "free" gift from our colleagues in tuberculosis control and the Expanded Programme on Immunization, but above all a fact.
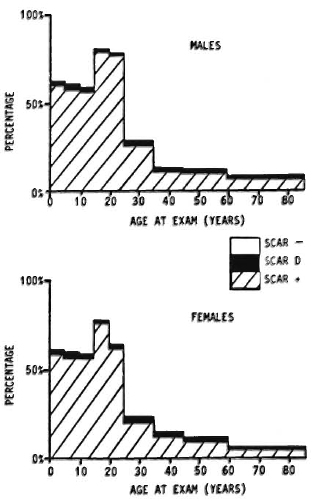
Fig. 4. Prevalence rates of BCG scars by age and sex in Karonga District, a rural population in northern Malawi (data from the Lepra Evaluation Project 14,61).
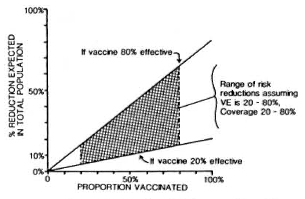
Fig. 5. Percent reduction (R) in overall incidence of disease as a function of vaccine efficacy (VE) and vaccine coverage (C) or proportion of the population vaccinated; relationship is R = C x VE.
Recognition of the inadequacies of BCG- the variability and unpredictability of its protective efficacy-has in recent years led to various attempts to improve upon it. Three of these approaches are now in phase 3 trials, as summarized in Table 6. One approach, based on a suggestion first proposed 30 years ago6 5 and drawing heavily upon the immunotherapy work of Dr. Convit in Venezuela, has been to attempt to increase the protection imparted by BCG by the addition of specific M. leprae antigens in the form of killed whole bacilli. The efficacy of combined BCG plus killed M. leprae vaccines is currently being assessed in field trials in Venezuela and in Malawi, and it is hoped that a third such trial may soon begin in India. The designs are slightly different, in that the Venezuela trial is based upon contacts of leprosy cases and uses different doses of BCG dependent upon prior tuberculin status;46 whereas the Malawi trial covers an entire district population and employs a single standard dose of BCG.66 Each trial uses plain BCG as a control group, and thus the results will be formulated in terms of the relative efficacy of the combined vaccines compared to BCG alone.
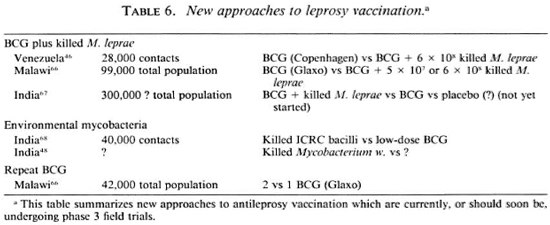
A different approach is currently being taken by some investigators in India, where killed environmental mycobacteria are being assessed as potential vaccines against leprosy. Of particular mention in this regard are the efforts of Dr. Deo and his colleagues with the so-called ICRC bacillus 68 and of Dr. Talwar and colleagues with the so-called Mycobacterium w.48
Another strategy has been to explore the implications of an even simpler approach to improve upon conventional BCG vaccination-merely repeating the BCG vaccination. We are used to the idea of booster inoculations for many, indeed most, vaccines, and repeated BCG vaccination has been standard policy in many countries for tuberculosis control. It is thus surprising that the implications of repeating a BCG vaccination have never been assessed up to this time. However, such studies are now underway on three continents and, interestingly enough, these studies are on leprosy not on tuberculosis. Although none of these studies has yet been published, preliminary analyses of the data from the BCG trial in Papua New Guinea by Bagshawe and colleagues6 9 and of case control studies in Venezuela by Drs. Convit, Smith and colleagues,70 each suggest a positive correlation between the numbers of BCG vaccinations and degree of protection. This hypothesis is built into the design of the Karonga Prevention Trial in northern Malawi, which will thus provide the first randomized controlled comparison ever carried out of one versus two BCG vaccinations in protecting against leprosy and tuberculosis.66
Discussion
In reviewing the subject of immunological tools in leprosy control, I have made no mention of several topics which are at the center of immunological research today, such as recombinant antigens, T-cell epitope mapping, and suppressor cells. These are tools to study how to make tools and do not, themselves, offer clear options for leprosy control, at least in the immediately foreseeable future. Someday they may lead to immense contributions by identifying antigens which induce protective immunity or by unraveling the mechanism of the human immune response to M. leprae, and hence leading to the development of diagnostic, therapeutic or preventative tools hardly dreamed of today. But that may take many years.
In the shorter term, we look at those tools already available or under trial -in particular serological tests for infection detection and disease classification, immunotherapcutic regimens, and new prophylactic vaccines-in the hope that some of these may soon join the list of applicable tools for leprosy control. None of them has yet proven itself, or been proven, to have such a role. But this should be taken as a challenge to their proponents, to demonstrate conclusively, and with appropriate appreciation of the harsh economic, logistic and social reality of leprosy control today, that these tools can so contribute.
For the present, we can appreciate our proven immunological tools, immunosuppressive drugs and BCG, which are playing an important role in treating and in preventing leprosy throughout the world today. They antedate the armadillo, but demonstrate the power of immunological interventions.
In referring to the present and future of immunology and leprosy, reference also needs to be made of the current global epidemic of HIV infection and AIDS. The prevalence of HIV infection is already high in many leprosy-endemic populations, and it will rise higher in the years to come. There has been much speculation about the implications of HIV infections for leprosy - that the destruction of T-cell -mediated immunity in affected individuals may lead to an increase in the incidence of leprosy or to a shift toward more multibacillary disease. A priori one might expect this, given the well-documented implications of HIV infections for other mycobacterial diseases.71 We might also expect that HIV infections will influence a variety of immunological interventions. There is a desperate lack of information on this issue thus far-but we cannot claim to have yet looked very hard for it. Aside from a few brief abstracts, there have been no published population studies on the relationship between HIV infection, AIDS and leprosy. Maybe most individuals with clinical AIDS and M. leprae infection die of other infections before leprosy manifests itself. We just don't know. Surely it must be included on our agenda in considering priorities in immunological research on leprosy and in considering the implications of immunological tools for leprosy control.
I have tried in this presentation to achieve a balance between promise and scepticism. The current wave of immunological activity promises much, but some will say it answereth not enough. One feels the restlessness from the field - the armadillo has been around long enough -it is time for results which count, for some new and practical contributions to leprosy control. Such impatience is understandable, but also needs to be tempered by the recognition that science is not the art of tackling the biggest problems but of tackling the soluble problems. The great advantage of a gathering such as this is that, by bringing together those scientists who are in a position to develop new tools with those who would use them, we have an opportunity to reorientate priorities; to decide which problems are not only soluble, but worth solving; to communicate; to facilitate the transfer of new technology from the laboratory to the field. This is all important, since there has been too little attention thus far to the tremendous problems of scaling an assay or an intervention up from its controlled application to 10 or 20 or 100 subjects or sera in a modern research facility to the huge logistic, economic and training implications of its application in the villages of leprosy-endemic populations. Indeed, there are too few basic scientists interested in this step, too few field researchers and projects able to mount the appropriate operational trials, and too few contacts between basic and applied research. Let us use this Congress to make those contacts. Let us get on with it.
- Paul E. M. Fine, V.M.D., Ph.D.
Department of Tropical Hygiene
London School of Hygiene and Tropical Medicine
Keppel Street
London WC1E 7HT, U.K.
Acknowledgments. Drs. A. C. McDougall, R. J. W. Rees and J. M. Ponnighaus made helpful comments on a draft of this presentation. Data in Figures 1-4 are based upon data collected in the Lepra Evaluation Project, which is funded primarily by LEPRA. Special thanks to A. Turner, and Dr. M. A. Shaw (A) and P. Burgess (B) for providing the ELISA results shown in Figure 3, and to Xenia Koning for preparation of the typescript.
1. Doull, J. A., Guinto, R. S., Rodriguez, J. N. and Bancroft, H. The incidence of leprosy in Cordova and Talisay, Cebu. P.I. Int. J. Lepr. 10(1942)107-131.
2. Fine, P. E. M. Leprosy-the epidemiology of a slow bacterium. Epidemiol. Rev. 4(1982)161-168.
3. White, S. J., Stone, M. M. and Howland, C. Genetic factors in leprosy: a studv of children in Uganda. J. Hyg.(Lond.)80(1978)205-216.
4. van Eden, W. and de Vries, R. R. P. Occasional review-HLA and leprosy: a re-evaluation. Lepr. Rev. 55(1984)89-104.
5. Fine, P. E. M. Implications of genetics for the epidemiology and control of leprosy. Philos. Trans. R. Soc. Lond. [Biol.] 1988(in press).
6. Taylor, C. E., Elliston, E. P. and Gideon, H. Asymptomatic infections in leprosy. Int. J. Lepr. 33(1965)716-727.
7. Ottenhoff, T. H. M. and de Vrics, R. R. P. HLA class II immune response and suppression genes in leprosy. Int. J. Lepr. 55(1987)521-534.
8. Convit, J., Pinardi, M. E., Arias Rojas, F., Gonzales, I., Corey, G., Arvelo, J. J. and Monzon, H. Tests with three antigens in leprosy-endemic and non-endemic areas. Bull. WHO 52(1975)193-198.
9. Pinto, M. R. M., Eriyagama, N. B. and Pemajayantha, V. Studies of reactivity of some Sri Lankan population groups to antigens of Mycobacterium leprae. II. Reactivity to a soluble protein antigen. Lepr. Rev. 58(1987)219-226.
10. Gupte, M. D., Nagaraju, B., Anantharaman, D. S. and Kannan, S. Use of soluble skin test antigens in leprosy. Abstract in Int. J. Lepr. 57 Suppl.(1989)000-000.
11. Zuniga, M. Use of skin tests with M. leprae soluble antigen for epidemiological studies and vaccination trials. Abstract in Int. J. Lepr. 57 Suppl.(1989)000-000.
12. Fine, P. E. M., Ponnighaus, J. M., Rees, R. J. W., Convit, J., Clarkson, J. A. and Bliss, L. Epidemiological studies with M. leprae soluble antigen skin tests. In: XII International Leprosy Congress Proceedings, New Delhi. February-20-25, 1984. Dcsikan, K. V., ed. New Delhi: PRINTAID, n.d., pp. 103-107.
13. Shield, M. J., Stanford, J. L., Garbajose, G., Draper, P. and Rees , R. J. W. The epidemiological evaluation, in Burma, of the skin test reagent LRA6; a cell-free extract from armadillo-derived Mycobacterium leprae. Part 1: Leprosy patients. Int. J. Lepr. 50(1982)436-445.
14. Ponnighaus, J. M., Fine, P. E. M., Bliss, L., Sliney, I. J., Bradley, D. J. and Rees, R. J. W. The Lepra Evaluation Project(LEP), an epidemiological study of leprosy in northern Malawi. I. Methods. Lepr. Rev. 55(1987)88-98.
15. Melsom, R. Serodiagnosis of leprosy: the past, the present and some prospects for the future. Int. J. Lepr. 51(1983)235-252.
16. Anonymous. Serological tests for leprosy. Lancet 1(1986)533-535.
17. Abe, M., Minagawa, F., Yoshino, Y., Ozawa, T., Saikawa, K. and Saito, T. Fluorescent leprosy antibody absorption(FLA-ABS)test for detecting subclinical infection with Mycobacterium leprae. Int. J. Lepr. 48(1980)109-119.
18. Cho, S. N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
19. Meeker, N. C, Levis, W. R., Scrsen, E., Schuller-Levis, G., Brennan, P. J. and Buchanan, T. M. ELISA detection of antibodies against phenolic glycolipid-I in the management of leprosy: a comparison of laboratories. Int. J. Lepr. 54(1986)530-539.
20. International Cooperative Team for Evaluating Serological Tests in Leprosy. A trial to compare serological tests for leprosy. Tokyo: Sasakawa Memorial Health Foundation, 1988, pp. 1-43.
21. Burgess, P. J., Fine, P. E. M., Ponnighaus, J. M. and Draper, C. C. The sensitivity, specificity and predictive value of ELISA tests based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidemiol. Inf.(1988)(in press).
22. Brett, S. J., Payne, S. N., Gigg. J., Burgess, P. and Gigg, R. Use of synthetic glyconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
23. Buchanan, T., Dissanayake, S., Young, D. B., Miller, R. A., Acedo, J. R., Harnisch, J. P., Khanolkar, S. R. and Estrada-Parra, S. Evaluation of the significance of antibodies to phenolic glycolipid of Mycobacterium leprae in leprosv patients and their contracts. Int. J. Lepr. 51(1983)658-659.
24. Chantcau, S., Cartel, J.-L., Guidi, C, Plichart, R. and Bach, M.-A. Scrocpidcmiological study on 724 household contacts of leprosy patients in French Polynesia using disaccharidc-octyl-BSA as antigen. Int. J. Lepr. 55(1987)626-632.
25. Douglas. J. T., Cellona, R. V., Abalos, R. M., Madarang, M. G. and Fajardo, T. Serological reactivity and early detection of leprosy among contacts of lepromatous patients in Ccbu, The Philippines. Int. J. Lepr. 55(1987)718-721.
26. Menzel, S., Harboe, M., Bergsvik, H. and Brennan, P. J. Antibodies to a synthetic analog of phenolic glycolipid-I of Mycobacterium leprae in healthy household contacts of patients with leprosy. Int. J. Lepr. 55(1987)617-625.
27. Klatser, P. R., de Wit, M. Y. L. and Kolk, A. H. J. An ELISA-inhibition test using monoclonal antibodv for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
28. Klatser, P. R., de Wit, M. Y. L., Cellona, R. V., Fajardo. T., Abalos, R. M. and Madarang, M. G. Serological activity to a 36 kDa antigen of M. leprae among household contacts of lepromatous patients. Abstract in Int. J. Lepr. 57 Suppl.(1989)000-000.
29. Sinha, S., Sengupta, U., Ramu, G. and Ivanyi, J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int. J. Lepr. 53(1985)33-38.
30. Ashworth, M., Sinha, S., Patil, S. A., Ramu, G. and Sengupta. U. The detection of subclinical leprosy-using a monoclonal antibody based radioimmunoassay. Lepr. Rev. 57(1986)237-242.
31. Mwatha, J., Moreno, C, Sengupta, U., Sinha, S. and Ivanyi, J. A comparative evaluation of serological assays for lepromatous leprosy. Lepr. Rev. 59(1988)195-199.
32. Blake, L. A., West, B. C, Lary, C. N. and Todd, T. R., IV. Environmental nonhuman sources of human leprosy. Rev. Infect. Dis. 9(1987)562-577.
33. Thomas, D. A., Mines, J. S., Thomas, D. C, Mack, T. M. and Rea, T. H. Armadillo exposure among Mexican-born patients with lepromatous leprosy. J. Infect. Dis. 156(1987)990-992.
34. Levis, W. R., Meeker, H. C, Schuller-Levis, G., Sersen, E. and Schwerer, B. IgM and IgG antibodies to phenolic glycolipid from Mycobacterium leprae in leprosy: insight into patient monitoring, erythema nodosum leprosum, and bacillary persistence. J. Invest. Dermatol. 86(1986)529-534.
35. Georgiev, G. D. and McDougall, A. C. The bacteriological examination of slit-skin smears in leprosy control programmes using multiple drug therapy: a plea for radical changes in current operational methodology. Indian J. Lepr. 59(1987)373-385.
36. Sanchez, G. A., Malik, A., Tougnc, C, Lambert, P. H. and Engers, H. D. Simplification and standarization of scrodiagnostic tests for leprosy based on phenolic glycolipid-I(PG-I)antigen. Lepr. Rev. 57 Suppl. 2(1986)83-93.
37. Brouard, Y. J., Blackwell, J. M. and Fine, P. E. M. Blood collection, fractionation and storage methods: a practical manual for immunocpidcmiological studies, with emphasis on work in developing countries(Dept. Trop. Hyg./LSHTM)(to be published).
38. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
39. Bach. M.-A., Wallach, D., Flagcul, B., Holfcnbach, A. and Cottenot, F. Antibodies to phenolic glycolipid-I and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
40. Miller, R. A., Gorder. D. and Harnisch, J. P. Antibodies to phenolic glycolipid-I during long-term therapv: serial measurements in individual patients. Int. J. Lepr. 55(1987)633-636.
41. Douglas, J. T., Steven, L. M., Fajardo, T., Cellona, R. V., Madarang, M. G., Abalos, R. M. and Stccnbcrgen, G. J. The effects of chemotherapy on antibody levels in lepromatous patients. Lepr. Rev. 59(1988)127-135.
42. Jacobson, R. R. Treatment. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 193-221.
43. Engers, H. and Ji, 13. Promotion of research on leprosy reactions and nerve damage. TDR News, 1988.
44. Schujman, S. Subsequent evolution of the induced Mitsuda reaction in clinically and bacterilogically negative lepromatous cases. Int. J. Lepr. 24(1956)51-56.
45. Lowe, J. and McNulty, F. The eflcct of BCG in lepromatous cases of leprosy. Int. J. Lepr. 21(1953)173-177.
46 Convit, J., Ulrich, M., Aranzazu, N., Castellanos, P. L., Pinardi, M. E. and Reyes, O. The development of a vaccination model using two microorganisms and its application in leprosy and leishmaniasis. Lepr. Rev. 57 Suppl. 2(1986)263-273.
47. Deo, M. G., Bapat, C. V., Bhalerao, V., Chaturvedi, R. M., Bhatki, W. S. and Chulawala, R. G. Antileprosy potentials of ICRC vaccine; a study in patients and healthy volunteers. Int. J. Lepr. 51(1983)540-549.
48. Talwar, G. P. Vaccine based on Mycobacterium w. bacillus. Working paper presented at "vaccines" workshop prior to XIII International Leprosy Congress, The Hague, The Netherlands, 1988.
49. Haregcwoin, A., Mustafa, A. S., Helle, I., Waters, M. F. R., Lciker, D. L. and Godai, T. Reversal by interlcukin-2 of the T-cell unresponsiveness of lepromatous leprosy to Mycobacterium leprae. Immunol. Rev. 80(1984)77-86.
50. Locniskar, M., McEniry, D. W., Mudd, D. W., Rose, P., Lucas. D. L., Larrick, J. and McAdam, K. P. W. J. Assessment of the immune deficit in leprosy patients and the effect of recombinant IL-2 vitro. Int. J. Lepr. 55(1987)249-260.
51. Nathan, C. F., Kaplan, G., Levis, W. R., Nusrat, A., Witmcr, M. D., Sherwin, S. A., Job, C. K., Horowitz, C. R., Steinman, R. M. and Cohn, Z. A. Local and systemic effects of intradermal recombinant in-terfcron-gamma in patients with lepromatous leprosy. N. Engl. J. Med. 313(1986)6-15.
52. Samuel, N. M., Grange, J. M., Samuel, S., Lucas, S., Owilli, O. M., Adalla, S., Leigh, I. M. and Navarrctte, C. A study of the effects of intradermal administration of recombinant gamma interferon in lepromatous leprosy patients. Lepr. Rev. 58(1987)389-400.
53. WHO Study Group. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
54. Truman, R. W., Morales, M. J., Shannon, E. J. and Hastings. R. C. Evaluation of monitoring antibodies to PGL-I in armadillos experimentally infected with M. leprae. Int. J. Lepr. 54(1986)556-559.
55. Stanley, S. J., Howland, C, Stoner, M. M. and Sutherland, I. BCG vaccination of children against lcprosv in Uganda: final results. J. Hvg.(Lond.)87(1981)233-248.
56. Scott, G. C, Russell, D. A., Boughton, C. R. and Vincin. D. R. Untreated leprosy: probability of shifts in Ridlcy-Jopling classification. Development of "flares," or disappearance of clinically apparent disease. Int. J. Lepr. 44(1978)110-122.
57. Lwin, K., Sundarcsan, T., Gyi, M. M., Bcchelli, L. M., Tamondong. C, Garbajosa. P. G., Sansarricq, H. and Noordccn. S. K. BCG vaccination of children against leprosy: fourteen-year findings of the trial in Burma. Bull. WHO 63(1985)1069-1078.
58. Tripathy. S. P. The case for BCG. Ann. Natl. Acad. Med. Sei.(India)19(1983)12-21.
59. Fine, P. E. M. The role of BCG in the control of leprosy. Ethiop. Med. J. 23(1985)179-191.
60. World Health Organization. Expanded programme on immunization. Update March 1988. Geneva: World Health Organization.
61. Fine, P. E. M., Ponnighaus, J. M. and Maine, N. The distribution and implications of BCG scars, with particular reference to a population in northern Malawi. Bull. WHO(in press).
62. Lechat, M. F., Vanderveken, M., Dcclcrq, E. and Misson, C. B. Analysis of trends in the occurrence of leprosy. World Health Stat. Quart. 39(1986)129-137.
63. Ponnighaus. J. M. and Boerrigter, G. Ten years' leprosy control work in Malawi (Central Africa)-II. Patterns of endemicity since 1973. Lepr. Rev. 57(1986)221-236.
64. Noordeen, S. K. and Lopez Bravo, L. The world leprosv situation. World Health Stat. Quart. 39(1986)122-128.
65. Hanks, J. H. and Fernandez, J. M. M. Enhancement of resistance to murine leprosy by BCG plus specific antigen. Int. J. Lepr. 24(1956)65-73.
66. Fine, P. E. M. and Ponnighaus, J. M. Background, design and prospects of the Karonga Prevention Trial(KPT), a leprosy vaccine trial in northern Malawi. Trans. R. Soc. Trop. Med. Hyg.(in press).
67. Gupte, M. D. Design and operational aspects: proposed leprosy vaccine trial -South India. Working paper presented at "vaccines" workshop prior to the XIII International Leprosy Congress, The Hague, The Netherlands. 1988.
68. Chaturvedi, R. M., Chirmule, N. B., Yellapurkar, M. V., Shaikh, S. V. and Deo, M. G. Effects of ICRC antileprosy vaccine in healthy subjects. Int. J. Lepr. 55(1987)657-666.
69. Bagshawe, A., Scott, G. C, Russell, D. A., Wigley, S. C, Merianos, A. and Berry, G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New-Guinea, 1963-1979(submitted for publication).
70. Convit, J. and Smith, P. G., personal communication, 1988.
71. Pitchenik, A. E., Fertel, D. and Bloch, A. B. Mycobacterial disease: epidemiology, diagnosis, treatment and prevention. Clin. Chest Med. 9(1988)425-441.
* Based on state-of-the-art lecture presented at the XIII International Leprosy Congress, 12 September 1988, The Hague, The Netherlands.