- Volume 57 , Number 3
- Page: 687–90
Demonstration of antidapsone antibody in leprosy patients
This department is for the publication of informal communications that are of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this JOURNAL is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the JOURNAL and thus interfere with its prime purpose.
To the Editor:
It well known that chemotherapy of leprosy with dapsone (DDS) is very effective but takes years to achieve a cure. Even for the tuberculoid type, treatment has to be continued for at least 2 years after the regression of the clinical lesion. Moreover, DDS is known to induce reactions (4,8) leading to deformities (10), and the patient may also become refractory to the drug. The exact cause of the reactions induced by DDS and the reason for its slow action and the fact that some patients do not respond to DDS therapy are not fully understood. However, nonresponsiveness to DDS therapy has been amply explained by the emergence of DDS-resistant strains of Mycobacterium leprae (9). It is known that DDS combines with many body proteins (1,2,6). In that case, the drug can act as a hapten with a distinct possibility to elicit an antibody response. These antibodies could neutralize biologically active units of the drug, leaving the patient with a much lower amount of the drug than indicated by crude blood level estimates. An inadequate dose of the biologically active drug may facilitate the emergence of resistant strains of M. leprae. These antibodies may also form DDS/ anti-DDS complexes which could contribute to the occurrence of reactions in leprosy. Before investigating these aspects, it should first be established if DDS therapy evokes an antibody response in the patients. In all, 178 subjects were studied. They included 140 leprosy patients on DDS therapy for at least 3 months, with a bacteriological status ranging from 0 to 5+. These patients included 71 cases of lepromatous or borderline lepromatous (LL/BL), 47 tuberculoid or borderline tuberculoid (TTV BT), 14 mid-borderline (BB), and 8 ncuritic types of leprosy. The 30 patients who were released from chemotherapy (RFC) during the preceding 12 months or more and who had maintained a negative bacterial status after stopping chemotherapy were also included. This group was composed of 15 cases of LL/BL, 5 TT/BT, 4 BB, and 6 neuritic types of leprosy. Eight normal controls were also included.
The proteins bovine serum albumin (BSA) or casein were conjugated to DDS by the diazotization reaction (11). Antibodies were raised in two albino rabbits by intradermal inoculation of an emulsion of 1 ml of DDSBSA (3 mg/ml) in 1 ml of Freund's incomplete adjuvant over 5 months with repeated booster injections every 3 weeks. One week after the last injection, the rabbits were bled and antibody titers were estimated. Fifty jug of this antibody (globulin fraction) was adsorbed with BSA, labeled with 125I (BARC, Bombay, India) employing the Iodogen (3) technique, and was used in the serum antibody competition assay (SACT) (7).
The mean binding of 125I-anti-DDS antibody to wells coated with casein alone was taken as 0%. This count was subtracted from each of the test binding values, and the relative binding values for each serum dilution were then calculated. Binding of 125I-anti-DDS alone to casein-DDS [80 μg/ml in phosphate buffered saline (PBS), pH 7.2] coated wells in the presence of cascin-PBS was taken as 100%. The results were expressed in terms of ID25 values which represent the serum dilutions causing 25% inhibition of 125I-anti-DDS antibody binding to the antigen (casein-DDS) coated wells. The cut-off point for positivity was calculated by adding two standard deviations (S.D.) to the mean ID25 value of the control sera.
The rabbit antisera showing the maximum titers (2000 and 3000 in the two rabbits, respectively) of anti-DDS antibody were pooled and used as an antibody source. These antibodies did not crossreact with other dapsone derivatives (DADDS, MADDS, diphenylsulfonc, sulfadiazine, etc.) (5). Anti-DDS antibodies were demonstrated in the globulin fraction rather than the whole serum of the leprosy patients. It was observed during standardization of the technique that the DDS present in the whole serum also competed with the antibody in the SACT assay. The globulin fractions of patient sera tested were found to be devoid of DDS and so were used for the SACT.
Six (13%) of the 47 TT/BT patients were positive for anti-DDS antibodies. In the BB leprosy patients, the anti-DDS antibody could be demonstrated in 4 (29%) of the total of 14 patients; of 71 LL/BL patients, 29 (41%) were antibody positive. The highest percentage (63%) of positive cases (5/8) was observed in the neuritic type of leprosy. Anti-DDS antibodies were absent in the 30 RFC cases and in the eight normal controls (Fig. 1). The mean ID25 titer in the TT/BT cases was 77, with a maximum of 200 in one of the patients. In the BB patients, the antibody titer ranged from 9 to 125. The mean antibody titers of LL/BL and neuritic patients were 194 (range ID25 8.8 to > 625) and 420 (range ID25 68 to > 625), respectively. A maximum antibody titer of > 625 was noticed in three LL/BL and two neuritic patients (Fig. 2). These antibodies were found to be of the IgG type, and had no correlation with the bacteriological status of the patients.
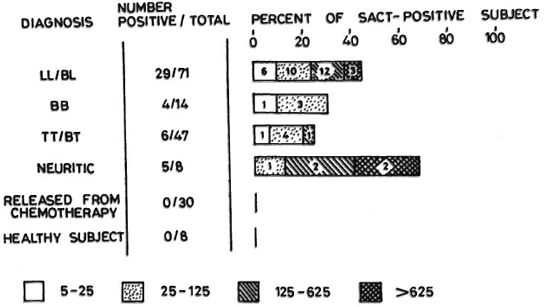
Fig. 1. Percent positivity and ID25 titers of anti-dapsone (DDS) antibody in leprosy patients. Sera from a total of 178 subjects were tested in duplicate at four log-5 serial dilutions by the SACT assay. Sera which failed to give 25% inhibition of 125I-anti-DDS antibody binding were taken as negative. Numbers of patients giving corresponding ID25 titers are shown within the bars.
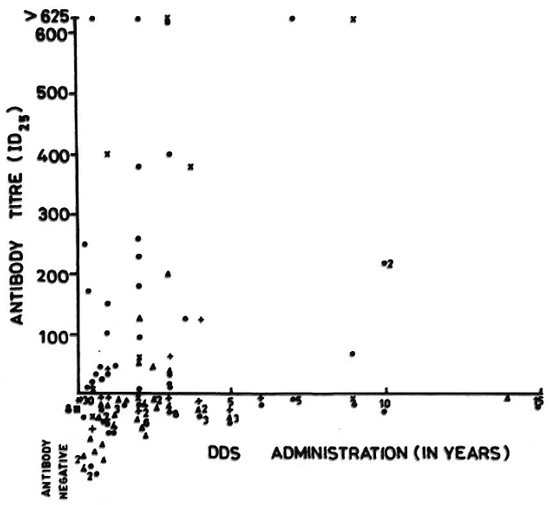
Fig. 2. Anti-DDS antibody titers (ID25) in DDS-treated leprosy patients.  = LL/BL;
= LL/BL;  = TT/BT; + = BB; = neuritic; * = released from control;
= TT/BT; + = BB; = neuritic; * = released from control;  = normal control.
= normal control.
The variations in the levels of antibody among the different types of leprosy patients may be due to genetic factors predisposing an individual to make an antibody response to a drug or may be related to the duration of chemotherapy. A patient not taking DDS and eight normal controls were never found to be positive for the anti-DDS antibody. Further studies are needed to find out the role of anti-DDS antibody in understanding the bioavailability of the drug, reactions, neuritis, and drug resistance in leprosy.
- Javed Nairn Agrewala, Ph.D.
Sudhir Sinha, Ph.D.
Satish K. Ghei, Ph.D.
Utpal Sengupta, Ph.D.
Department of Immunology
- Kiran Katoch, M.D.
Bhawneshwar K. Girdhar, M.D.
Clinical Division
Central JALMA Institute for Leprosy
Agra 282 001, India
Acknowledgments. We thank Dr. H. Srinivasan for providing valuable suggestions during the preparation of the manuscript. We also thank Mr. M. M. Alam and K. K. Kulshrestha for technical assistance, Mr. Hari Om Agarwal for photography, and Mr. Anil Kumar Chopra for secretarial help.
REFERENCES
1. BIGGS, J. T. and LEVY, L. Binding of dapsone and monacetyldapsone by human plasma proteins. Proc. Soc. Exp. Biol. Med. 137(1971)692-695.
2. DATTA, G., NAIK, S. S. and GURNANI, S. Chemical characterization of the dapsone binding site of lysozyme. Int. J. Lepr. 53(1985)428-432.
3. FRAKER, P. J. and SPECK, J. C, JR. Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3α,6αdiphenylglycoluril. Biochem. Biophys. Res. Common. 80(1978)849-857.
4. FREY, H. M., GERSUON, A. A., BORKOWSKY, W. and BULLOCK, W. E. Fatal reaction due to dapsone during treatment of leprosy. Ann. Intern. Med. 94(1981)777-779.
5. HlJIKESHOVEN, H., DE WlT, M., SOETERS, A., EGGELTE, T. A., LANDHEER. J. E. and LEIKER, D. L. ELISA inhibition technique for the demonstration of sulphones in body fluids. I. Sulphones specific antibody-enzyme conjugate. Lepr. Rev. 50(1979)275-281.
6. RILEY, R. W. and LEVY, L. Characteristics of the binding of dapsone and monoacetyldapsone by serum albumin. Proc. Soc. Exp. Biol. Med. 143(1973)1168-1170.
7. SINHA, S., SENGUPTA, U., RAMU, G. and IVANYI, J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int. J. Lepr. 53(1985)33-38.
8. SMITH, W . C. S. Are hypersensitivity reactions to dapsone becoming more frequent? Lepr. Rev. 59(1988)53-58.
9. SUBCOMMITTEE ON CLINICAL TRIALS OE THE CHEMOTHERAPY OF LEPROSY (THELEP) SCIENTIFIC WORKING GROUP OF THE UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. Characteristics of multiplication of dapsone-resistant strains of Mycobacterium leprae in mice. Lepr. Rev. 59(1988)5-10.
10. WARDEKAR, R. V. Sulphone treatment and deformity in leprosy. Lepr. India 40(1968)161-178.
11. WILLIAMS, C. A. and CHASE, M. W., eds. Methods in Immunology and Immunochemistry. New York: Academic Press, 1967, vol. 1, pp. 135-145.
Reprint requests to Dr. Sengupta.