- Volume 57 , Number 3
- Page: 690–2
Serum antibodies of normals and leprosy patients show equal binding to peripheral nerve
To the Editor:
The presence of serum dcmyclinating factors (SDF) may lead to disseminated nerve damage in leprosy despite the infection being localized. Over the past few years several workers have detected the presence of SDFs in leprosy by using either binding assays to detect serum antibodies against peripheral nerve antigens (4,7) or through functional assays where demyclination is observed by electron-microscopic examination of the sciatic nerve of Swiss white mice after an intraneural injection of test serum (9).
Nonetheless, it is imperative to locate and characterize these SDFs in leprosy patients. The assay adopted by Shetty, et al. (9) is obviously cumbersome for screening a-large number of sera. Hence, the prescrcening of sera using an ELISA was devised with the purpose of eliminating those samples having the least binding toward human peripheral nerve antigens. The serum demonstrating the highest affinity could then be used later for the functional assay. This communication describes the screening assay utilized and discusses the implications of the results obtained.
Antigen. Normal human nerve (posterior tibial and sural) was collected from a freshly amputated limb in sterile Hanks' balanced salt solution. The epineurium was removed and the nerve was incubated at 37ºC for 30 min in 0.05% collagenase solution (Collagenase Type II; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) prepared in Dulbecco's modified Eagle medium (GIBCO Laboratories, Grand Island, New York, U.S.A.). Thereafter the nerve was cut, finely chopped, and sonicated in 2.5% sodium dodccyl sulfate for 2 hr with 30 sec pulses. After centrifugation at 500 rmp for 10 min, the supernatant was assayed for protein content (2). Aliquots (100 μl) of the supernatant from a single nerve sample were stored at - 20ºC and used for the entire assay. The supernatant was diluted with phosphate buffered saline (PBS), pH 7.4, to give a final concentration of 500 ng per 50 μl per well.
Antibody. Human serum was diluted 1:10 using 5% BSA (bovine serum albumin, fraction V; Loba Chemie, Bombay, India) in PBS. Leprosy patients were classified according to the Ridley-Jopling scale (8). The Figure contains the details of the subjects studied.
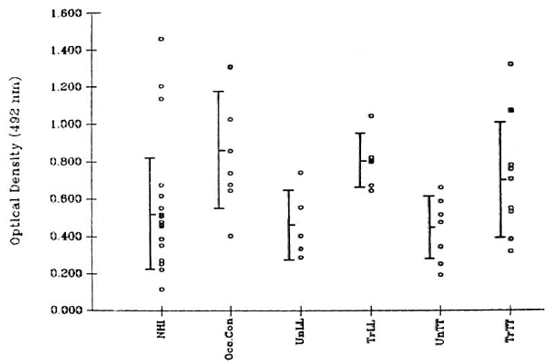
The figure. Binding values ( ) for each serum sample were calculated by subtracting the optical density (OD) at 492 nm of wells that were not coated with antigen from that obtained in wells where the test serum was added to antigen-coated wells. In the absence of primary antibody (human serum samples), the OD values at 492 nm did not exceed 0.15. A marginally significant (p < 0.05) difference was observed between the binding activity of sera from 18 normal healthy individuals (NH I) and that of 8 occupational contacts (Occ.Con) consisting of laboratory personnel. The difference in serum binding activity between NHI and leprosy patients was not significant. Lepromatous leprosy patients included 5 untreated (UnLL), 4 treated (TrLL), and 1 patient who was treated and was undergoing an erythema nodosum leprosum reaction at the time of sample collection (
) for each serum sample were calculated by subtracting the optical density (OD) at 492 nm of wells that were not coated with antigen from that obtained in wells where the test serum was added to antigen-coated wells. In the absence of primary antibody (human serum samples), the OD values at 492 nm did not exceed 0.15. A marginally significant (p < 0.05) difference was observed between the binding activity of sera from 18 normal healthy individuals (NH I) and that of 8 occupational contacts (Occ.Con) consisting of laboratory personnel. The difference in serum binding activity between NHI and leprosy patients was not significant. Lepromatous leprosy patients included 5 untreated (UnLL), 4 treated (TrLL), and 1 patient who was treated and was undergoing an erythema nodosum leprosum reaction at the time of sample collection ( ). Tuberculoid leprosy patients included 8 untreated (UnTT), 8 treated (TrTT), and 1 treated patient currently experiencing a reaction (upgrading?) (
). Tuberculoid leprosy patients included 8 untreated (UnTT), 8 treated (TrTT), and 1 treated patient currently experiencing a reaction (upgrading?) ( ).
).
Conjugate. Peroxidase-conjugated rabbit immunoglobulin to human IgG (gammachain) (DAKO-Immunoglobulins, Denmark) was diluted 1:2000 using 5% BSA in PBS.
Substrate composition. Four mg orthophenylencdiaminc (Sigma) was dissolved in 10 ml citrate phosphate buffer (pH 5.0) and 4 μl of hydrogen peroxide was added just before use.
ELISA conditions. A modified method of Cho, et al. (3) was employed for the enzyme-linked immunosorbent assay. The antigen was added to NUNCLON U-bottom, 96-well plates (NUNC, Denmark) and incubated at 37ºC for 72 hr in a moist chamber. The wells were washed with PBS 6 times, and blocked by the addition of 100 μl of PBS containing 1% BSA at 37ºC for 1 hr in a moist chamber. The contents were aspirated, and 50 μl of the diluted serum (1:10) was added. The plates were incubated at 37ºCfor 1 hr, and washed with PBS 10 times, followed by the addition of conjugate (50 μl). After a 1-hr incubation at 37ºC, the wells were washed 15 times with PBS, and 50 μl of the substrate was added. The reaction was terminated with 2.5 N sulfuric acid after incubating the plates in the absence of light for 20 min. The adsorbance was read at 492 nm using a Titertek Multiskan Plus (Flow Laboratories, Finland).
The results shown in The Figure depict equal binding of serum antibody from normals and leprosy patients to human peripheral nerve sonicate. A marginally significant difference (p < 0.05) was observed between the binding activity of sera from normal healthy individuals and occupational contacts. There was no difference in binding between sera of patients throughout the disease spectrum, and patients undergoing reactions did not exhibit values different from those obtained by the rest of the group. A similar pattern of results was observed earlier in experiments using a sonicate of mouse sciatic nerve as the antigen coat (unpublished observations).
The significant amount of serum antibody detected in normals against peripheral nerve antigens may be due to the presence of natural antibodies against neural glycolipid antigen as depicted by ccrcamide pentasaccharide (6). In patients, however, the specificity of antibodies for peripheral nerve may be due to these natural antibodies as well as antibodies directed against mycobacterial components that may crossreact with peripheral nerves. Preliminary experiments have demonstrated the marked binding activity of anti-BCG serum (Dakopatts, Copenhagen, Denmark) to the same human peripheral nerve antigen coat used in the above-mentioned experiments. Similar crossreactivity of sera from leprosy patients) was also observed between a 35-kDa neural antigen and a synthetic analog of the terminal disaccharide portion of phenolic glycolipid-I (1).
This test as described above therefore fails to distinguish sera that can be used in functional demyclination assays. Nevertheless, these findings may have implications regarding the suitability of this or other-similar tests that have been utilized recently for the detection of early leprosy (5).
- Percy S. Ghaswala, M.Sc.
Research Assistant
(Senior-Project)
- Nerges F. Mistry, Ph.D.
Senior Research Officer
- Noshir H. Antia, F.R.C.S., F.A.C.S.(Hon.)
Director and Trustee
The Foundation for Medical Research
84-A R.G. Thadani Marg
Worli
Bombay 400018, India
Acknowledgments. This work was supported by a grant from the Department of Science and Technology, New Delhi. We gratefully acknowledge Dr. Pervez Hakim and the resident doctors of the Orthopedic Department, Sir J.J. Group of Hospitals, Bombay, for providing us with human nerves. We also wish to thank Mr. Rane of St. George's Hospital Blood Bank for the supply of normal sera and the authorities of the Acworth Leprosy Hospital for providing us with patient sera.
REFERENCES
1. BENJAMINS, J. A., CALLAHAN, R. E., RUNFT, D., GERRAS, G. and LEFFORD, M. J. Anti-neural antibodies in leprosy sera: further characterization of the antigens. J. Neuroimmunol. 21(1989)125-135.
2. BRADFORD, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochcm. 72(1976)248-254.
3. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
4. EUSTIS-TURF, E. P., BENJAMINS, J. A. and LEFFORD, M. J. Characterization of the anti-neural antibodies in the sera of leprosy patients. J. Ncuroimmunol. 10(1986)313-330.
5. ITTY, B. M., MUKHERJEE, R. and TALWAR, G. P. An enzyme immunoassay(EIA)based on antibodies against human nerve antigen for diagnosis of all categories of leprosy patients. Abstract in Int. J. Lepr. 57Suppl.(1989)304.
6. KUROIWA, A., IGISU, K., YANO, T., OKADA, N. and OKADA, H. Fibroncctin enhances respiratory burst of phagocytes stimulated by zymosan and immune complexes. Immunol. 65(1988)177-180.
7. MSHANA, R. N., HARBOE, M., STONER, G. L., HUGHES, R. A. C, KADLUBOWSKI, M. and BELEHU, A. Immune responses to bovine neural antigens in leprosy patients. I. Absence of antibodies to an isolated myelin protein. Int. J. Lepr. 51(1983)33-40.
8. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
9. SHETTY, V. P., MISTRY, N. F. and ANTIA, N. H. Serum demyclinating factors and adjuvant-like activity of Mycobacterium leprae: possible causes of early nerve damage in leprosy. Lepr. Rev. 56(1985)221-227.