- Volume 57 , Number 2
- Page: 451–7
Results of a modified WHO regimen in highly bacilliferous BL/LL patients
ABSTRACT
A regimen consisting of 600 mg of rifampin once a month, 100 mg of clofazimine on alternate days, and 100 mg of dapsone daily was used in 56 untreated, highly bacillated borderline lepromatous/lepromatous (BL/LL) patients with an average bacterial index (BI) of 4.45. Treatment was continued until skin-smear negativity. After 2 years of therapy, none of the patients had become smear negative and the average BI was 2.56. There was no growth on inoculation of skin-tissue biopsies in the normal mouse foot pad after 6 months of therapy. Bacillemia was still detectable in 11/50 patients, and significant ATP levels were detected in Mycobacterium leprae from skin-tissue biopsies in 16% of the cases. After 3 years of therapy, three patients had become smear negative. The average BI was 1.30. None of the patients had detectable bacillemia, and 5% of the cases showed detectable ATP levels in M. leprae from tissue biopsies. After 4 years of therapy, 41.7% of the patients had become smear negative. The average BI was 0.66, and no ATP was detected in any of the purified bacillary suspensions. The fall in BI was accelerated, and more patients on continued treatment became negative earlier compared to those having treatment for a limited duration, as reported by others.RÉSUMÉ
Chez 56 malades atteints de lèpre lÚpromateuse dimorphe ou de lèpre lépromateuse (BL/LL), hautement bacillifère, ayant un Index Bactériologique (BI) de 4,45, on a utilisé une posologie consistant en l'administration de 600 m g de rifampicine une fois par mois, de 100 mg de clofazimine tous les deux jours, et de 100 mg de dapsone quotidiennement. Le traitement a été poursuivi jusqu'à ce que les frottis deviennent négatifs. Après deux années de traitement, aucun des malades ne présentait des frottis négatifs, et l'Index Bactériologique moyen était de 2,56. On n'a pas constaté de croissance des bacilles après inoculation de biopsies cutanées dans le coussinet plantaire de la souris normale, après 6 mois de traitement. Chez 11 malades sur 50, on pouvait encore déceler une bacillémie, et des niveaux significatifs d'ATP ont été décelés dans 16% des cas chez des Mycobacterium leprae recueillis à partir de biopsies cutanées. Après 3 ans de thérapie, trois malades présentaient des frottis négatifs. L'Index Bactériologique moyen était de 1,30. Aucun des malades n'avait alors de bacillémie décelable, et 5% des cas montrait des niveaux décelables d'ATP dans M. leprae provenant de biopsies de tissu. Après 4 ans de traitement, 41,7% des malades étaient devenus négatifs. L'Index Bactériologique moyen était de 0,66, et l'ATP n'a été mis en évidence dans aucune des suspensions purifiées de bacille. La chute de l'Index Bactériologique était accélérée; contrairement à ce qui a été rapporté par d'autres auteurs, la proportion de malades devenant négatifs plus précocement était supérieure à ce qui est constaté après un traitement de durée limitée.RESUMEN
Se usó un esquema de tratamiento consistente en 600 mg de rifampins una vez al mes, 100 mg de clofa/imina cada tercer día, y 100 mg de dapsona diarios, en 56 pacientes altamente bacilíferos (índice bacteriológico. I.B. de 4.45) sin tratamiento. Se intentó continuar el tratamiento hasta negativi/ar los extendidos de piel pero después de 2 años de terapia, ninguno de los pacientes llegó a ser negativo y el I.B. promedio fue de 2.56. Sin embargo, cuando se inocularon los cojinetes plantares de ratones normales con material de las biopsias de piel obtenidas después de 6 meses de terapia, no hubo crecimiento bacilar. La bacteremia fue aún detectable en 11 de 50 pacientes y en el 16% de los casos se delectaron niveles elevados de ATP en los Mycobacterium leprae del tejido de las biopsias de piel. Después de 3 años de terapia, los extendidos de piel de 3 pacientes se tornaron negativos para bacilos. El I.B. promedio fue de 1.30. Ninguno de los pacientes tuvo bacilcmia détectable y el 5% de los casos tuvo niveles medibles de ATP en los M. leprae de los tejidos de las biopsias. Después de 4 años de terapia, el 41.7% de los pacientes fueron negativos para bacilos. El I.B. fue de 0.66 y no se detectó ATP en ninguna de las suspensiones de bacilos purificados. Comparando los resultados del presente estudio de tratamiento continuo con los reportados por otros de tratamiento limitado, se observa que la caída en el I.B. fue acelerada y más pacientes se tornaron bacteriológicamente negativos en forma más temprana.Multidrug therapy (MDT) has been advocated in all cases of multibacillary leprosy for several reasons. Firstly, the problems of primary and secondary drug resistance could be prevented. Secondly, by using a combination of drugs with different modes of action, Mycobacterium leprae are attacked at different stages of their life cycle and thus could be more effectively killed. Thirdly, in cases who have already become dapsone resistant, only a combination of drugs would be advisable. A World Health Organization (WHO) Study Group has recommended a multidrug regimen (17) for 2 years or longer for multibacillary patients. Different workers in leprosy have also tried different multidrug regimens and have advocated treatment for varying durations for achieving the most effective bacterial kill and for minimizing relapses. There have been only a few reports of long-term follow-up of the effects of multidrug treatment on highly bacilliferous leprosy patients or of detailed studies on the results of MDT regimens (3,6,11,16).
We have earlier reported (6) the preliminary results of a 2-year follow-up of highly bacillated multibacillary patients on a modified WHO regimen. The present study now reports the results of those cases who have been followed up for 4 years. The regimen used was the same as the WH O regimen except that no monthly supervised loading doses, i.e., 300 mg of clofazimine, were given and clofazimine was administered 100 mg on alternate days.
PATIENTS AND METHODS
Fifty-six untreated borderline lepromatous (BL) and lepromatous (LL) patients between the ages of 18 and 45 years who attended the outpatient department of the Central JALMA Institute for Leprosy and who had a bacterial index (BI) of 4 to 6 + (Ridley scale 14) were the subjects of this study. All but three of these patients were males. The regimen used in the present study is: rifampin 600 mg once a month, supervised; clofazimine 100 mg on alternate days, unsupervised; and dapsone 100 mg daily, unsupervised. Treatment was continued until smears became negative.
Before starting treatment, these patients had been examined in detail and the findings were carefully recorded on a body chart along with the clinical score (5). Smears were taken from four different skin sites and the BI (14) and morphological index (MI) (10) were calculated. Nasal smears were also taken from all of the patients. In a few patients, a biopsy was performed for histopathology. The lepromin test had been done in a majority of the patients. Bacillemia was assessed by the hemolytic method (13) and was estimated every 6 months up to 3 years.
Skin biopsies were done for all these patients, for inoculation into the foot pads of mice, at the beginning of treatment and then every 6 months. The biopsy specimen was minced with scissors, homogenized, and suspended in Hanks' balanced salt solution, carrying out all procedures ascptically at low temperature over ice. After allowing the suspensions to stand for 3 min, the supernatant fluid was collected and the bacterial enumeration was carried out as described by D'Arcy Hart and Rees (1). A batch of six randomly bred Rockefeller mice were inoculated into each hind foot pad with a 0.03 ml suspension containing up to 104 bacilli. The inoculated mice were housed at 25ºC in an air conditioned room. One mouse was harvested every month beginning 6 months after inoculation by the method described by Desikan and Venkataramanaiah (2), and the bacilli were counted. The percentage of viable pcrsistcrs being low in the long-treated cases, even a 10-fold increase in the harvest count was taken as true multiplication for all these studies.
Patients were examined every month or, in some cases, every 2 months. They were carefully examined at each visit and their clinical progress was noted. At the end of every 6 months of therapy, bacillemia was estimated, their clinical score reassessed, and a skin biopsy obtained for animal inoculation. Smear examinations were repeated from the same sites as the original ones and their BI and MI were recorded. ATP measurements were made from the skin-biopsy specimens at 24 months, 36 months, and 38-45 months after initiation of MDT by the method of Katoch, et al. (6).
RESULTS
The average BI at the start of therapy was 4.45; the average MI was 1.66% (range 1%9%). The MI fell rapidly in the initial 6 months of therapy. At 6 months the MI was 1% in only one patient; in all the others, it was zero. After 6 months of MDT, only one patient was positive for acid-fast bacilli (AFB) in a nasal smear as compared to 20 at the start of therapy. The fall in the average BI was very gradual in the first year of therapy. It fell by 0.67 at the end of 1 year; thereafter the fall in BI was greater. Part of these results have been published elsewhere (6).
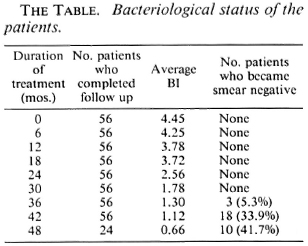
The Table shows the details of the average BI at different periods after starting MDT and also the number of patients who became smear negative at different time intervals. It can be seen that the BI fell from the initial 4.45 to 0.66 at 48 months, and 3 out of 56 (5.3%), 18 out of 53 (33.9%), and 10 out of 24 (41.7%) became smear negative after 36 months, 42 months, and 48 months of therapy, respectively.
The clinical scores were calculated by the method described by Iyer, et al. (5). The average clinical score was 16 at the start of therapy. Clinical regression of lesions was most rapid in the first year of therapy. All nodules and plaques regressed in all of the patients in the initial 12 months. The average clinical score was 7 at the end of 1 year. Subsequently, the infiltration and thickening of skin regressed more gradually. There was loss of skin elasticity and residual thickening of the skin persisted even after the smears had become negative.
Ten patients were in erythema nodosum leprosum (ENL) reaction before the start of MDT. During the MDT period, there was no increase in the incidence of reactions. In the few patients who did develop ENL and/ or neuritis during therapy, the reaction was of mild-to-moderate severity and was easily controlled by steroids and injections of sodium antimony gluconate. Only one patient had a severe pustulating ENL reaction requiring thalidomide for its control. Specific treatment for leprosy was not stopped during reaction in any of the cases.
Bacillemia was estimated by the hemolysis method at six monthly intervals in all of the patients. At the start of therapy, 28 of the 53 patients showed bacillemia. Bacillemia was observed in 11 patients even after 2 years of therapy, but after 30 months of treatment bacilli could not be demonstrated in the blood of any of the patients. These results have been reported earlier (6).
Bacilli from all of the patients were inoculated into the foot pads of normal mice at the start of therapy, and multiplication was observed in 54 out of 56. At the end of 6 months of therapy, bacilli from only one patient showed multiplication in the mouse foot pad and thereafter no multiplication in the mouse foot pad was seen in any of the biopsies. After 24 months of therapy, mouse foot-pad inoculation could not be done in many cases because the bacillary counts had become very low.
Skin biopsies were obtained from the patients after 2 and 3 years of MDT, and the ATP content was estimated by the method of Katoch, et al. (7). After 2 years of MDT, 5 out of 32 patients (16%) had appreciable ATP levels in the bacilli purified from their tissue biopsies; at 3 years, 1 out of 20 patients (5%) still had detectable ATP levels in their bacillary suspensions. Detectable ATP levels were not observed in 13 patients who had completed 38 to 45 months of treatment and were still skin-smear positive.
DISCUSSION
The advent of MDT has raised the hopes for cure of leprosy patients with a limited duration of treatment. In our study, we have used a regimen which is not very different from the regimen advocated by the WHO Study Group (17). In this present regimen only the supervised monthly loading dose of 300 mg of clofazimine was not given and clofazimine was administered 100 mg on alternate days. For practical purposes, this regimen is comparable to that recommended by the WHO Study Group. The Indian Association of Leprologists (IAL) has advocated continuous rifampin administration for the first 21 days of treatment followed by the same WHO regimen. Therapeutically, this IAL regimen does not seem to offer any distinct advantage over the WHO regimen (3). The THELEP trials of WHO included five different regimens of which two consisted of MDT for 3 months only followed by daily dapsone, and one regimen consisted only of a single dose of rifampin followed by daily dapsone therapy. THELEP has combined the results of all of these five regimens and reports the results collectively. However, we have attempted a comparison of those results with ours (Fig. 1).
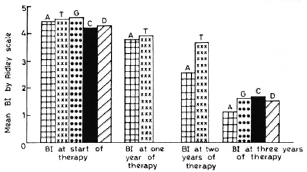
Fig. 1. Result of average 131 in different regimens at start of therapy and 1, 2, and 3 years.  A = present regimen (modified WHO);
A = present regimen (modified WHO);  T = combined results of all five THELEP regimens (15);
T = combined results of all five THELEP regimens (15);  G = results of study of Ganapati, et al. (3);
G = results of study of Ganapati, et al. (3);  C = rifampin-containing regimen of Thomas, et al. (16);
C = rifampin-containing regimen of Thomas, et al. (16);  D = nonrifampin regimen of Thomas, et al. (16).
D = nonrifampin regimen of Thomas, et al. (16).
Bacterial index. In the present study, the mean BI fell gradually from an initial average of 4.45. At the end of 2 years it was still 2.56. It was 1.3 and 0.66 at the end of 3 and 4 years, respectively. The mean BI at the start of therapy in the THELEP trials (15) was 4.8 for Bamako subjects and 4.3 for subjects from Chingleput. The THELEP report gives the combined results of all the regimens in both of these places. The mean BI has been reported to be 4.42, 3.98, and 3.67, respectively, at 3 months, 12 months, and 24 months after starting therapy. Thus, this BI was one log higher than our results obtained after 2 years of MDT. Ganapati, et al. (3) have tried both the WHO advocated regimen and the IAL regimen in untreated, highly bacillated, multibacillary patients. Since the IAL regimen did not offer any distinct advantage, they have combined the results of both regimens and reported an average BI of 1.67 at the end of 3 years of MDT, which is similar to our results. Thomas, et al. (16) reported on two MDT regimens: one containing rifampin, isoniazid, clofazimine, and dapsone in which rifampin and isoniazid are given for the first 3 months followed by clofazimine and dapsone, and the other being a nonrifampin regimen consisting of clofazimine and dapsone only. By 3 years of therapy, the average BI was 1.72 and 1.56, respectively, for the rifampin and nonrifampin regimens. So it appears that under an effective MDT regimen the mean BI falls slowly but steadily to around 1.5 by 3 years of therapy, irrespective of minor differences in the regimens (Fig. 1).
Achievement of negativity. The WHO Study Group has advocated stopping MDT in multibacillary cases when the skin smears become negative or after 2 years, whichever is later. When untreated, highly bacilliferous BL/LL cases were studied, it was observed that none of the cases became negative by 2 years of MDT (3,6,15,16). There are a few reports (8,12) indicating that smear negativity was attained in a significant proportion within 2 years of MDT. These reports, however, relate to patients who had an average BI of less than 3 at the time of starting MDT.
By 3 years of continuous MDT, none of the patients studied by Thomas, et al. (16) became smear negative. Ganapati, et al. (3) reported that 25 out of 90 patients (28%) became negative in their study in the same duration. In the present study 3/56 patients (5.3%) became negative after 3 years, 18/56 (33.9%) after 3½ years, and 10/24 (41.7%) after 4 years of MDT. These results show that the highly bacillated untreated group of BL/LL patients start to become negative by 3 years of MDT but it will probably take 5 to 6 years for the entire group to become negative.
Pattyn, et al. (11) have reported their results in highly bacillated cases with two limited-duration regimens (1 year of treatment followed by a placebo). Since duration of therapy is an important factor influencing patient compliance, it will be interesting to compare our results with those of Pattyn, et al., although their trial includes a relatively small number of patients. The initial Bis in their two regimens (P1 and P2) at the time of starting treatment were 4.5 and 4.3, respectively, and compare with ours (details in Fig. 2). After 1 year, i.e., at the time of stopping treatment, the average BI had not fallen, being 4.6 and 4.0, respectively. At the end of 3 years, the average BI in regimen PI was 4.1, nearly the same as the original value (4.5), and none of the patients had become negative. In regimen P2 of Pattyn, et al. the mean BI had fallen from 4.3 to 3.1 in 3 years, but none of the patients had become negative. By 4 years of follow up, only 1 out of 29 (3%) patients had become negative and the average BI was 2.7. In contrast, in our study with treatment continued, 41.7% of the patients had become smear negative and the average BI was only 0.66 at 4 years. This suggests that continued treatment results in a much steeper fall in BI (Fig. 2), and the attainment of skin-smear negativity is also much faster.
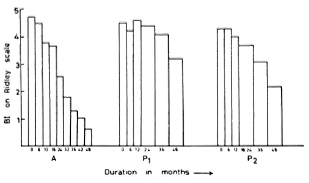
Fig. 2. Fall in BI of present continuous regimen and two limited-duration regimens of Pattyn, et al. after 4 years of therapy. A = WHO modified regimen reported in this paper; P1 = regimen A of Pattyn, et al. (11) consisting of rifampin 600 mg twice a week x 6 months + DDS 100 mg daily x 1 year; P2 = regimen B of Pattyn, et al. (11) consisting of rifampin 600 mg twice a week x 6 months + prothionamide 500 mg daily x 6 months + DDS 100 mg daily x 1 year.
Viability. With MDT a large proportion of the bacilli are killed very rapidly in the initial stages. Inoculation of skin biopsies into the normal mouse foot pad shows that infectivity to normal mice is lost very rapidly, and by the first 6 months of therapy, there was multiplication in only 1 out of 54 biopsies inoculated. Subsequently, there was no multiplication in the mouse foot pad. In the THELEP trials in Chingleput and Bamako (15) where thymectomized-irradiated (TR) mice were used, persisting M. leprae were detected in about 9% of the cases after 2 years of MDT. When there are very few viable organisms in a relatively large population of dead bacilli, the normal mouse foot pad model is not suitable for demonstrating the presence of those few viable bacilli (9). This situation occurs within a few months of MDT when there is no multiplication in the normal mouse foot-pad model but the presence of pcrsisters can be demonstrated by TR mice even after 2 years of MDT, as in the THELEP study (15) and as reported in neonatally thymectomized rats by Gelber, et al. (4).
To detect the small number of viable organisms, we processed the tissue-biopsy specimens for ATP estimation by a method which can detect even small numbers of colony-forming units of cultivable mycobacteria (7). It was observed that by 2 years of MDT, 16% of our patients showed appreciable ATP levels in the bacilli purified from their tissue biopsies. This figure fell to 5% with continuation of treatment for another year, i.e., at 3 years. Significantly, no detectable ATP levels were present in the purified bacilli from cases who had completed 38-45 months of treatment (manuscript in preparation). It may be mentioned here that when ATP estimations are made in untreated patients, the bacilli from their tissues have viable populations in all (100%) and the levels fall with MDT (manuscript in preparation). Thus, both the THELEP studies and the present ATP studies show that, even after 2 years of therapy, at least about 10% to 15% of the patients continue to show the presence of viable bacilli. The persistent absence of bacillemia when patients are under continued therapy suggests that there probably are no foci left from which bacilli are being liberated into the bloodstream. This appears likely because values of viability indicators, such as the MI, mouse foot pad results, and ATP estimations, also progressively decline. With the present knowledge, all of these findings suggest that in the highly bacillated BL/LL cases it would be advisable to continue treatment beyond 2 years until the achievement of smear negativity.
Acknowledgments. The authors are grateful to Dr. H. Srinivasan. Director, for his constructivo comments; Mr. J. D. Kushwaha for secretarial help; and Mr. Hari Om for artistic help in preparing the manuscript. The technical assistance by Surender K. Bhan is also gratefully acknowledged.
REFERENCES
1. D'ARCY HART. P. and REES, R. J. W. Effect of macrocyclon in acute and chronic pulmonary tuberculosis infection in mice as shown by viable and total bacterial count. J. Exp. Pathol. 41(1960)414-120.
2. DESIKAN, K. V. and VENKATARAMANAIAH, H. N. A modified method of harvesting M. leprae from foot pads of mice. Lepr. India 48(1976)157-162.
3. GANAPATI, R., REVANKAR, C. R. and PAI, R. R. Three years assessment of multidrug therapy in multibacillary leprosy cases. Indian J. Lepr. 59(1987)44-49.
4. GELBER, R. H., HUMPHRES, R. C. and FIELDSTEEL, A. H. Superiority of the neonatally thymectomized Lewis rat (NTLR) to monitor a clinical trial in lepromatous leprosy of the two regimens of rifampin and dapsone. Int. J. Lepr. 54(1986)273-282.
5. IYER, C. G. S., BALAKRISHNAN, S. and RAMU, G. A comparison of low and conventional dosage of dapsone in treatment of lepromatous leprosy. Lepr. India 49(1977)372-386.
6. KATOCH, K., RAMU, G., RAMANATHAN, U., SENGUPTA, U. and SREEVATSA. Follow-up of BL/ LL patient on a slightly modified WHO regimen of multidrug therapy. Indian J. Lepr. 59(1987)36-43.
7. KATOCH, V. M., KATOCH, K, RAMU, G., SHARMA, V. D., DATTA, A. K. SHIVANNAVAR, C. T. and DESIKAN, K. V. In vitro methods for determining the viability of mycobacteria: comparison of ATP content, morphological index and FDA-EB fluorescent staining in Mycobacterium leprae. Lepr. Rev. 59(1988)137-143.
8. KAUR, S., SHARMA, V. K, KUMAR, B., SINGH, M. and KAUR, I. Multidrug therapy in bacilliferous leprosy; two years experience. Indian J. Lepr. 57(1985)483-490.
9. LEVY, L. Application of the mouse foot pad technique in immunologically normal mice in support ofclinical drug trials and a review ofcarlierclinical drug trials in lepromatous leprosy. Int. J. Lepr. 55 Suppl.(1987)823-829.
10. MCRAE, D. H. and SHEPARD. C. C. Relationship between the staining quality of Mycobacterium leprae and infectivity in mice. Infect. Immun. 3(1971)116-120.
11. PATTYN, S. R., SAINT ANDRÉ, P., FERRACI, C. and BAQUILLON, G. Comparative study of two regimens of combined chemotherapy of one year duration in multibacillary leprosy. Results after four and five years of follow-up. Int. J. Lepr. 52(1984)297-303.
12. RANGARAJ, M. and RANGARAJ, J. Experience with multidrug therapy in Sierra Leone: clinical, operational and managerial analysis. Lepr. Rev. 57 Suppl. 3(1986)77-91.
13. RAVAL, S. N., SENGUPTA, U., RAMU. G., PRABHUNE, P. V. and DESIKAN. K. V. A study of continuous bacillaemia in borderline and lepromatous type of leprosy. Lepr. India 54(1982)623-633.
14. RIDLEY. D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Bristol: John Wright and Sons Ltd., 1964, pp. 620-622.
15. SUBCOMMITTEE ON CLINICAL TRIALS OF THE SCIENTIFIC WORKING GROUP ON CHEMOTHERAPY OF LEPROSY (THELEP) OF UNDP/WORLD BANK/ WHO SPECIAL PROGRAMME FOR RESEARCH IN TROPICAL DISEASES. The THELEP controlled clinical drug trials. Int. J. Lepr. 55 Suppl.(1987)864-868.
16. THOMAS, A., BALAKRISHNAN, A., N AGARAJAN, M., PRADHAKAR, R. and TRIPATHY, S. P. Controlled clinical trial of two regimens in multibacillary cases of leprosy: An interim report. In: Proceedings of XII International Leprosy Congress, New Delhi, 1984. Desikan, K. V., ed. Delhi: n.d., pp. 254-255.
17. WHO STUDY GROUP. Chemotherapy of Leprosy for Control Programmes. Geneva: World Health Organization. 1982. Tech. Rep. Ser. 675.
1. M. D., Senior Research Officer; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
2. M.D., Former Deputy Director; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
3. M.B.B.S., D.P.M., Assistant Research Officer; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
4. Ph.D., Deputy Director; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
5. Ph.D., Research Officer; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
6. M.Sc, Assistant Research Officer; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
7. M.Sc, Research Assistant, Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
8. M.D., Assistant Director, Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
Dr. Ramu's present address: Senior Physician, Sacred Heart Hospital, Kumbakonam, Tamil Nadu, India.
Received for publication on 27 September 1988.
Accepted for publication in revised form on 14 November 1988.