- Volume 57 , Number 2
- Page: 458–64
Relapses in paucibacillary patients after treatment with three short-term regimens containing rifampin
ABSTRACT
Three multidrug regimens all containing rifampin and dapsone have been tried for the treatment of 278 cases of paucibacillary leprosy. Regimen I was the one recommended by the WHO Study Group. Regimen II was the same as Regimen I with depsone alone continued for a further 6 months. Regimen III was the same as Regimen II but rifampin was given daily for the first 7 days. The patients were comparable with regard to disease classification, lepromin status, bacteriological status, and number of lesions. As reported earlier, the disease inactivity rates by 1 year of treatment were much greater with Regimens II and III than with Regimen I (94% and 97% vs 76%).Early reaction was seen in 6% of those in Regimen III and in none in Regimens I and II. Late reaction was observed in 9% of those in Regimen I and none in Regimens II and III. During 3½ years of follow up, 13% of the cases in Regimen I, 1% in Regimen II, and 2% in Regimen III relapsed. Since the patients in the three regimens were otherwise comparable, it is concluded that the high inactivity rate, low relapse rate (1% 2%), and no early or late reaction as observed in Regimen II patients were because of adequate treatment.
RÉSUMÉ
Chez 278 cas de lèpre paucibacillaire, on a procédé à un essai de traitement comparant trois posologies de polychimiothérapic, contenant toutes trois de la rifampicinc et de la dapsone. La posologie I était celle recommandée par le Groupe d'Etude de l'OMS. La posologie II était la même que la première, mais avec 6 mois supplémentaires de dapsone seule. La posologie III était la même que la deuxième, sauf que la rifampicine était administrée quotidiennement pendant les 7 premiers jours. Les malades étaient comparables en ce qui concerne la classification de la maladie, la réaction à la lépromine. l'état bactériologique, et le nombre de lésions. Ainsi qu'on l'a rapporté antérieurement, les taux d'inactivation de la maladie après un an de traitement étaient beaucoup plus élevés avec les posologies II et III qu'avec la posologie I (respectivement 94% et 97%. contre 76%).Une réaction a été constatée précocement chez 6% des malades soumis à la posologie III. mais chez aucun des malades auxquels avaient été administrées les posologies I et II. Une réaction a été observée plus tardivement chez 9% des malades recevant la posologie I. et chez aucun des malades recevant les posologies II et III. Au cours des 3½ années de suivi, 13% des malades soumis à la posologie I ont récidivé, ces taux étant de 1% chez les malades recevant la posologie II et de 2% chez les malades ayant été traités par la posologie III. Puisque les patients exposés à ces trois posologies étaient comparables à tout autre égard, on en conclut que le taux élevé d'inactivation, ainsi que le faible taux de récidive (1%-2%), de même que le fait qu'aucune réaction précoce ou tardive n'a été notée, démontrent qu'il s'agit là d'un traitement adéquat.
RESUMEN
Se estudió la eficiencia de 3 esquemas de tratamiento con múltiples drogas, todos ellos conteniendo rifampina y dapsona. usados en 278 casos con lepra paucibacilar. El esquema I fué el recomendado por la Organización Mundial de la Salud (WHO). El esquema II fué el mismo que el 1 pero se continuó con dapsona sola durante 6 meses adicionales. El esquema III fué el mismo que el esquema II pero con rifampina administrada diariamente durante los primeros 7 días. Los pacientes fueron comparables con respecto a clasificación de la enfermedad, reactividad a la lepromina, estado bacteriológico, y número de lesiones. Como se reportó antes, un año después de iniciado el tratamiento el grado de inactividad de la enfermedad fué significativamente mayor con los esquemas II y III que con el esquema I (94% y 97% vs 76%).La reacción temprana se observó en el 6% de los casos tratados con el esquema III y en ninguno de los casos tratados con los esquemas I y II. La reacción tardía se observó en el 9% de los casos sometidos al esquema I y en ninguno de los casos tratados con los esquemas II y III. Durante el seguimiento por 3 años y medio, se observó que recayeron el 13% de los casos en el esquema I, el 1% en el esquema II, y el 2% en el esquema III. Puesto que los pacientes en todos los casos fueron comparables, se concluye que el alto grado de inactividad, la baja freeuencia de recaída (1%-2%) y la ausencia de reacciones temprana y tardía observadas en los pacientes tratados con el esquema II, se deben a lo adecuado del tratamiento.
Paucibacillary (PB) leprosy patients constitute 70% to 80% of the total leprosy patients in India. These patients require a minimum of 4½ to 6 years of dapsone monotherapy before they can be declared as cured and released from control (16). Such a long period of treatment leads to poor patient compliance and a high dropout rate. The advent of multidrug therapy (MDT) has created the possibility of shortening the duration of treatment and thus improving patient compliance. The WHO Study Group has advocated MDT with rifampin and dapsone for 6 months for PB leprosy patients. Only a few reports are available of the relapse rates in PB patients after MDT. In this study we have compared the efficacy of three MDT regimens containing rifampin. Information regarding attaining inactivity following these regimens has been reported earlier (9,10). We report here the relapse rates and results of follow up of these cases for 3-3½ years after stopping MDT.
PATIENTS AND METHODS
Three-hundred-twenty patients with PB leprosy registered at the JALMA Hospital during 1983-1984 were the subjects of this study. The results of 236 patients who were available for 1½ years of follow up have been published earlier (10). Subsequently, 42 patients (of the same 320 patients) who simultancously had completed the regular treatment along with the above patients could be contacted. They were followed up for 3-3½ years and have been included in the present analysis. Therefore, a total of 278 patients had been followed up for 3 to 3½ years and the results are presented here. The study subjects were in the age group of 20-25 years and included 63 women patients. They were all previously untreated (so far as could be ascertained from history and occasional urine examinations) and had indeterminate (I), tuberculoid (TT), or borderline tuberculoid (BT) leprosy diagnosed clinically. Their bacterial index (BI) ranged from 0 to 2, according to the Ridley scale, and they conformed to the criteria for PB leprosy laid down bv a WHO Study Group (18).
The skin lesions were recorded on body charts and areas of anesthesia were also mapped. Skin-smear examination for acid-fast bacilli (AFB) was done initially in all cases, and this was repeated at the end of therapy in those who were positive earlier. Smear examinations were also done from lesions appearing active at the end of therapy or during the follow-up examinations. The cutaneous nerve thickening was marked on the patient's body chart at the start of therapy and assessed at every visit. Sensory motor assessment was done as and when required. The Mitsuda test was done for all patients, using Dharmendra antigen, before starting treatment, and was considered positive when the intradermal nodule measured 4 weeks later was 5 mm or more in diameter.
The patients were serially allotted to Regimens I, II or III.
Regimen I. (WHO Study Group regimen). Rifampin 600 mg once a month supervised x 6 months; dapsone 100 mg daily unsupervised x 6 months. Treatment was stopped at the end of 6 months.
Regimen II. Rifampin 600 mg once a month supervised x 6 months; dapsone 100 mg daily unsupervised x 1 year. Treatment was stopped at the end of 1 year.
Regimen III. Rifampin 600 mg supervised daily x 7 days in the first month followed by rifampin 600 mg once a month x 5 more months; dapsone 100 mg daily unsupervised x 1 year. Treatment was stopped at the end of 1 year.
All patients were put on placebo tablets after stopping the specific treatment.
After termination of chemotherapy, the patients were seen once every 3 months or earlier if their clinical conditions warranted. During the follow-up period the patients were assessed regarding disease activity, reaction, relapse, nerve involvement, and nerve damage. These terms have been defined earlier (10) and are detailed below.
Reaction. The term reaction is defined as a sudden and abrupt appearance of erythema, swelling and tenderness in the whole of the existing lesion(s) with or without the appearance of new lesion(s) with similar signs of inflammation. In some patients, the reaction was associated with systemic symptoms of fever and malaise; in a few others there was ulceration of the lesions. Reaction was often associated with tenderness in the nerves.
Early reaction was diagnosed when the signs and symptoms of reaction appeared during the course of therapy. Late reaction was diagnosed when it occurred up to 12 months after stoppage of therapy.
Relapse. Relapse was diagnosed when signs and symptoms of disease activity appeared after the complete subsidence of the disease. The onset was more gradual and insidious. Activity appeared in a few of the old lesions from all or part of the margin with or without the appearance of new lesions. These lesions showed no signs of inflammation. This was not associated with any systemic disturbance, and there was no tenderness in the nerves. In a few patients, the smears from such lesions became positive.
RESULTS
The details of the type of leprosy, the Mitsuda test, smear positivity, and number of lesions in these groups of patients were given in our earlier publication (10).
It may be noted that differences in the distribution of the subjects according to type of disease among the different regimens are not statistically significant (x2 = 0.8345, p > 0.5). Regimen I included 24 TT, 63 BT, and 8 I patients. Regimen II was composed of 20 TT, 58 BT, and 10 I patients. Regimen III was composed of 22 TT, 62 BT, and 11 I patients.
The late lepromin (Mitsuda) responses of the patients were more or less similar among the patients in different regimens (x2 = 0.0524, p > 0.5). Of the 95 patients of Regimen I, Mitsuda responses were positive in 71 and negative in 12; results were not available in 12. In Regimen II, 65 were lepromin positive, 10 were negative, and lepromin testing could not be done in the remaining 13. Regimen III included 71 lepromin-positive and 10 lepromin-negative subjects; results were not available in the remaining 14. The distribution of bacteriologically positive cases was not significantly different in the three regimens (x2 = 0.2211, p > 0.5): 20 of 95 in Regimen I, 18 of 88 in Regimen II, and 22 of 95 in Regimen III were bacteriologically positive, showing occasional bacilli with a BI of < 2 on the Ridley scale.
The patients were analyzed according to the number of lesions. In Regimen 1,41 had 1-5 lesions, 30 had 6-10 lesions, and the remaining 24 had 11 or more lesions. In Regimen II, 43 had 1-5 lesions, 27 had 6-10 lesions, and 18 had > 11 lesions. In Regimen III, 32 had 1-5 lesions, 33 had 6-10 lesions, and 30 had 11 or more lesions. The distribution of these cases among the different regimens is not significantly different (x2 = 5.0589, p > 0.1).
Table 1 shows the relapse rate and the incidence of reactions in these patients. None of the patients of Regimens I and II developed "early reaction" but 7 of the 95 patients in Regimen III had "early reaction." In all of these cases, the reaction subsided during the treatment period and none developed deformities. Nine patients on Regimen I had "late reaction"; in seven of them the reactions occurred during the first 6 months after stopping therapy. In the remaining two, these reactions occurred during the 7th to 12th month of follow up. The "late reaction" had a sudden onset with swelling, inflammation, and erythema in all the lesions. New lesions developed in four patients. It was interesting to note that apart from one instance ofdevclopment ofa nerve abscess which regressed without leaving any deformity, there was no other incidence of nerve damage in cases with late reaction, possibly because of the early start of anti-reaction treatment. Late reaction was not observed in any of the patients in Regimens II and III.
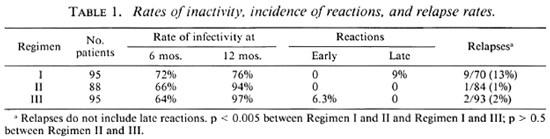
Twenty-five patients on Regimen I, four patients on Regimen II, and two patients on Regimen III remained active at the time of stoppage of therapy and their details have already been published (10).
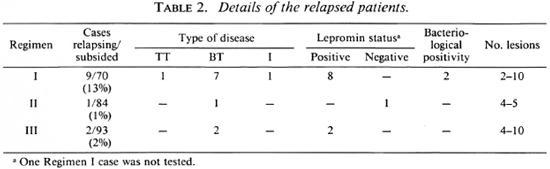
Table 2 give the details of relapse in the three groups ofpatients in whom the disease had subsided with chemotherapy. It should be mentioned that cases of "late reaction" have not been included in "relapses." Relapses occurred more often in patients on Regimen I than in those on Regimens II or III (13% vs 1% and 2%, respectively). Statistical evaluation showed that this difference was highly significant (p < 0.005).
DISCUSSION
During the dapsone monotherapy era, paucibacillary (PB) patients were advised to continue treatment for at least 2 years after they became clinically inactive or for a minimum period of 4½ years (16). The relapse rate after dapsone monotherapy was reported to be related to the number of lesions (4), duration of therapy (15), type of leprosy (2) and also, to some extent, lepromin positivity (6,7).
With the advent of multidrug therapy (MDT) it is hoped that the duration oftreatment will be reduced and patient compliance will be increased. For any therapy to be considered effective, the relapse rates should also be at a minimum acceptable level. Only a few reports are available regarding relapse rates after MDT. The WHO Sixth Expert Committee on Leprosy (17) reported a global relapse rate of 1 per 1000 during 12-37 months of posttreatment surveillance. However, Samuel, et al. (14) from Nepal have reported a relapse rate of 9% in smear-negative PB patients and of 13% in smear-positive PB patients in 18 months of follow up after 6 and 10 months of MDT, respectively. Pavithran (12) from India observed a relapse rate of 12% in patients under the WHO Study Group regimen during 12 months of posttreatment follow up. In the present study, we have the results in 278 patients ofthree short-term two-drug regimens, all containing rifampin. Although the allocation to the different regimens was done serially and not randomly, it was observed that the patients on the different regimens were comparable regarding parameters which could influence patient responses, such as type of leprosy, bacterial positivity, number of lesions, and the Mitsuda response.
The rates of inactivity in the three regimens have been published earlier (10). To summarize the results, about 65%-70% of the patients in all three regimens became inactive by 6 months of therapy. Similar results have also been reported by other workers (1,3,8,11,13). By year the rate was much greater with Regimens II and III where treatment was stopped at 6 months (94% and 97% vs 76%, respectively). Although 18 of the 25 active cases of Regimen I required rctreatment, none of the remaining active cases from Regimens II or III required further treatment.
Early reaction was not observed in patients on Regimens I and II, but 6% of those on Regimen III developed early reaction. This was the group which had received the initial daily rifampin for 7 days but otherwise had the same treatment as those in Regimen II. It appears that such initial treatment with daily rifampin, while conferring no benefit, might have even contributed to the occurrence of early reactions.
Distinguishing "relapse" from "late reaction" can be very difficult in PB leprosy, and there are no universally accepted definitions of these terms. For the purpose of our study we have defined "late reaction" as mentioned earlier, i.e., appearance of erythema and tenderness in all of the existing lesion(s) often associated with tenderness in the nerves and with or without the appearance of new lesion(s). This was often accompanied by other local and systemic evidence of acute inflammation. Late reaction, according to these criteria, occurred up to 1 year after stopping treatment and was observed in nine (9%) patients on Regimen I. No patient on Regimens II or III developed late reaction. It appears that continuing dapsone therapy for a further 6 months can avoid the problem of "late reaction." This is very important since reactivation of the lesions, whether it is called reaction or relapse, would surely contribute to the morbidity in these patients.
Relapse as defined earlier, i.e., appearance of disease activity in previously subsided lesions plus or minus new lesions, occurred in 9 patients (13%) (3 in first year, 5 in second year, and 1 in third year of follow up) who were on Regimen I compared to 1 (1%) and 2 (2%) of the patients in Regimens II and III, respectively. It must be stressed that we have calculated the relapse rate only for those patients who were at risk of relapse, i.e., those who had clinically subsided at the time ofstopping therapy. The relapses occurred insidiously with an increase in the size of some of the old lesions and without any systemic symptoms. In some patients a few new lesions appeared. None of the le-sions showed any ulceration or desquamation, indicating the absence of acute inflammation. In two patients the margins of the relapsed lesions showed occasional bacilli.
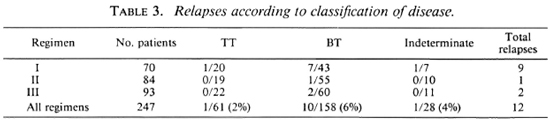
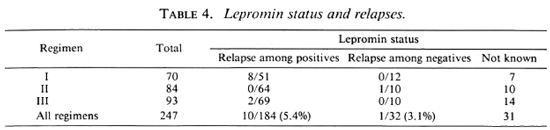
In this study we found that relapses could not be correlated with type of disease (Table 3), lepromin status (Table 4), initial bacteriological status (Table 5), or number of lesions (Table 6).
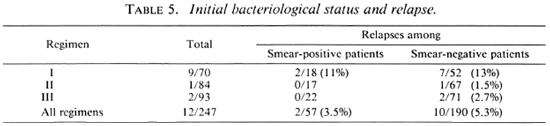
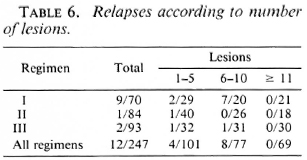
The difference in the relapse rates between Regimens II and III was not statistically significant (p > 0.5). However, the differences in relapse rates between Regimens I and II and Regimens I and III were highly statistically significant (p < 0.005). Since the patients in these three groups were otherwise comparable, the differences in the relapse rates could be attributed to the different treatment schedules. Supplementing the WHO regimen with 6 more months of dapsone therapy (Regimen II) effectively reduced the relapse rate to acceptable limits and also eliminated both early and late reactions. The recommendations of the National Leprosy Programme in India (5) state that smear-negative PB patients should be treated with the WHO Study Group regimen for 6 months and more until the patients become clinically inactive. The present study shows that even in patients who had clinically subsided with 6 months of MDT, the incidence of late reaction and the relapse rate were very high and these could be successfully overcome by continuing dapsone therapy for a further 6 months.
Jesudasan, et al. (6,7) have reported that lepromin-positive patients had a lower relapse rate under dapsone monotherapy than lepromin-negative patients. (The lepromin testing was done at the time of release from control.) However, we find in this study that with MDT the initial lepromin positivity did not influence the relapse rate (Table 4). Similarly, other factors, such as bacterial positivity, number of lesions, and type of disease, taken individually, did not influence the relapse rate (Tables 3-6).
To conclude, late reaction and relapse were observed (10% to 15% each) in patients treated by the WHO Study Group regimen. It is prudent that all PB patients be treated for 1 year because when the WHO Study Group regimen was supplemented with 6 more months of unsupervised dapsone therapy, there were no early or late reactions and the relapse rate was low (1%-2%). We could not demonstrate any influence individually of factors such as lepromin status, bacteriological positivity, classification of disease, and number of lesions on the relapse rates. Administration of daily rifampin for 7 days in the first month did not offer any advantage, and so it is not advised.
Acknowledgments. The authors are thankful to Dr. H. Srinívasan, Director, for his constructive comments and review of the manuscript. The secretarial assistance of Mr. J. D. Kushwah is gratefully acknowledged.
REFERENCES
1. BHATE, R. D., GUPTA, C. M., CHATTOPADHYAY, S. P. and SINGH, I. P. Experience with multidrug therapy in paucibacillary leprosy. Indian J. Lepr. 58(1986)244-250.
2. DAVEY, T. F. "Release from control" in leprosy. (Editorial) Lepr. Rev. 49(1978)1-6.
3. DHIR, R., GUHA, P. K. and SINGH, G. Short term chemotherapy of paucibacillary leprosy. Indian J. Lepr. 58(1986)549-554.
4. EKAMBARAM, V. Duration of treatment for "disease arrest" of non-lepromatous cases and relapse rate in these patients. Lepr. Rev. 50(1979)297-302.
5. Guidelines on Case Detection, Treatment, Follow-up and Reporting. National Leprosy Eradication Programme. New Delhi: Directorate General of Health Services, 1987, pp. 16-17.
6. JESADUSAN, K., CHRISTIAN, M. and BRADLEY, D. Relapse rates among nonlepromatous patients released from control. Int. J. Lepr. 52(1984)304-310.
7. JESUDASAN, K. and CHRISTIAN, M. Risk of paucibacillary leprosy patients released from control relapsing with multibacillary leprosy. Int. J. Lepr. 53(1985)19-21.
8. KAR, P. K., JHA, P. K., PANAYACH, J. S. and SNEHI, P. S. A clinico-pathological study of multidrug regimen in paucibacillary leprosy. Indian J. Lepr. 60(1988)235-241.
9. KATOCH, K., RAMU, G. and RAMANATHAN, U. Comparison of three regimens containing rifampicin for treatment of paucibacillary leprosy patients; a preliminary report. Indian J. Lepr. 57(1985)499-506.
10. KATOCH, K., RAMU. G., RAMANATHAN, U. and DESIKAN, K. V. Comparison of three regimens containing rifampin for treatment of paucibacillary leprosy patients. Int. J. Lepr. 55(1987)1-8.
11. KAUR, S., SHARMA. V. K., BHUSHAN, K. and KAUR. 1. Experience with two drug regimen in paucibacillary leprosy; a preliminary report. Indian J. Lepr. 56(1984)48-19.
12. PAVITHRAN, K. Relapse of paucibacillary leprosy after short course multidrug therapy. Indian J. Lepr. 60(1988)225-229.
13. REVANKAR, C. R., GANAPATI, R. and NAIK. D. D. Multidrug therapy for paucibacillary leprosy: experience in Bombay. Indian J. Lepr. 57(1985)773-779.
14. SAMUEL, N. M., SAMUEL. S. and ADIGA, R. B. Treatment of paucibacillary leprosy patients with dapsone and rifampicin. Jpn. J. Lepr. 54(1985)193-197.
15. TOUW LANGENDIJK, E. M. J. and NAAFS, B. Relapses in leprosy after release from control. Lepr. Rev. 50(1979)123-127.
16. WHO Expert Committee on Leprosy. Fourth Report. Geneva: World Health Organization, 1970. Tech. Rep. Ser. 495.
17. WHO Expert Committee on Leprosy. Sixth Report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
18. WHO Study Group. Chemotherapy of Leprosy for Control Programmes. Geneva: World Health Organization, 1982, 24-27. Tech. Rep. Ser. 675.
1. M. D., Senior Research Officer; Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India
2. M.B.B.S., D.P.M., Assistant Research Officer; Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India
3. M.B.B.S., D.V.D., Research Officer; Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India
4. M.B.B.S., Research Officer; Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India
5. M.Stat., Senior Research Officer; Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India
6. M.Phil., Research Assistant; Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India
7. M.D., formerly Deputy Director, Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India
Dr. Ramu's present address: Senior Physician, Sacred Heart Hospital, Kumbakonum, Tamil Nadu, India.
Received for publication on 14 November 1988.
Accepted for publication in revised form on 1 1 January 1989.