- Volume 57 , Number 2
- Page: 506–10
An electron microscopic study of lymphatics in the dermal lesions of human leprosy
ABSTRACT
The dermal lymphatic vessels in lepromatous and tuberculoid leprosy lesions were studied by light- and electron-microscopy. In the lepromatous patient, lymphatic vessels were seen in both intra- and peri-granulomatous areas. The lymphatic lining cells contained lipid droplets, lysosomes, and numerous pinocytotic vesicles. Cells bearing bacilli were only occasionally seen. In the tuberculoid cases, lymphatic vessels were seen only along the edges of the granulomas and the lining cells were less prominent. Inflammatory cells, both lymphocytes and histiocytes, were found traversing the walls of lymphatic vessels in both groups of patients. The results of the study confirm the continued and increased functioning of the lymphatic drainage system in dermal leprosy lesions, and indicates that it may be a major route for the clearance of lipids f rom the lipid-rich bacilliferous lesions in the lepromatous patient. The lymphatic pathway appears to bc a minor pathway for the dissémination of Mycobacterium leprae in comparison with the blood vascular System.RÉSUMÉ
On a étudié par la microscopie optique et par la microscopic électronique les vaisseaux lymphatiques du derme dans des lésions de lèpre lépromateusc et de lèpre tuberculoide. Chez le malade lépromatcux, on a observé des vaisseaux de lymphatiques tant dans les zones intragranulomateuses que dans les zones périgranulomatcuscs. Les cellules du revêtement lymphatique contenaient des gouttelettes de lipide, des lysosomes, et de nombreuses vésicules pinocytoliques. Ce n'est que rarement qu'on a relevé la présence de cellules contenant des bacilles. Dans les cas de lèpre tuberculoide, on a noté la présence de vaisseaux lymphatiques seulement le long des bords des granulomes; les cellules de revêtement étaient moins visibles. Dans les deux groupes de malades, des cellules inflammatoires, consistant en lymphocytes et histiocytes, traversaient les parois des vaisseaux lymphatiques. Les résultats de cette étude confirment la persistance et l'augmentation du fonctionnement du système de drainage lymphatique dans les lésions dermiques de la lèpre; ils indiquent également que ce système pourrait constituer chez le malade lépromatcux une voie importante pour l'évacuation des lipides des lésions bacillifères. Le réseau lymphatique semble jouer un rôle moindre que le système vasculairc sanguin dans la dissémination de Mycobacterium leprae.RESUMEN
Utilizando las microscopías de luz y electrónica, se estudiaron los vasos linfáticos dérmicos en las lesiones de la lepra lepromatosa y tuberculoide. En las lesiones lepromatosas se observaron vasos linfáticos en las áreas intra-y perigranulomatosas. Las células de revestimiento linfático contuvieron gotas de lípidos, lisosomas, y numerosas vesículas pinociticas. Sólo ocasionalmente se observaron células con bacilos. En los casos tuberculoides, los vasos linfáticos sólo se observaron en los bordes de los granulomas y las células de revestimiento fueron menos prominentes. En ambos grupos de pacientes se observaron células inflamatorias (linfocitos c histiocitos) atravezando las paredes de los vasos linfáticos. Los resultados del estudio confirman el continuo funcionamiento del sistema de drenaje linfático en las lesiones de la lepra, e indican que ésta puede ser una importante ruta de depuración de los lípidos de las lesiones lepromatosas bacilíferas ricas en lípidos. Comparado con el sistema vascular sanguíneo, el sistema linfático representa sólo una ruta menor de diseminación del Mycobacterium leprae.Involvement of regional lymph nodes in both lepromatous and tuberculoid leprosy is seen quite frequently (4,5,9,10,14). The histopathological changes in lymph node lesions are similar to those in the skin (4). While these observations indicate a regular flow of bacterial antigens/bacteria through the lymphatic system, there is as yet no description of the changes in the dermal lymphatic capillary network around the granuloma. The present study describes the fine structural changes occurring in these vessels in leprosy lesions.
MATERIALS AND METHODS
Skin biopsies were collected from eight lepromatous (LL and BL) and eight tuberculoid (TT and BT) leprosy patients. The tissues were immediately fixed in Trump's fixative (4% formol-1% glutaraldehyde in phosphate buffer, pH 7.2) for 24 hr and then processed for embedding in Spurr's resin after postfixing in 1% osmium tetroxide. Initially, 1-μm semithin sections were cut and stained with 1% toluidinc blue in borax for scanning and identification of sections with lymphatic vessels. Selected blocks were then final trimmed, and ultrathin sections (gold to silver) were cut using glass knives and double contrasted with uranyl acetate and Reynold's lead citrate. Finally, the sections were examined under a JEOL 100 CXII electron microscope.
The patients included in the study were attending the Leprosy Clinic at the Department of Dermatology, Safdarjang Hospital, New Delhi, India. There was no history of previous treatment for leprosy in any of the patients. None of the cases, lepromatous or tuberculoid, showed any features of type 1 or type 2 reactions at the time the biopsy was taken.
RESULTS
Light-microscopic examination of the 1-μm semithin sections showed that initial lymphatics could definitely be identified in the subpapillary dermis in these sections. The lymphatics had irregularly shaped lumina and thin walls consisting of a single layer of endothelial cells without a well-defined basement membrane or pericytes being seen around them (Fig. 1). Using these criteria, lymphatics were identified within the granuloma and in the dermal collagen around the granulomas in the lepromatous lesions. In the tuberculoid lesions, the lymphatics were identified only in the perigranulomatous areas. In both groups, lymphatics in the sections were predominantly located on the edge of the granuloma. The lumen was seen in all of these lymphatics, and appeared to be dilated in some of them.

Fig. 1. Photomicrograph of lymphatic vessel adjacent to dermal granuloma in BT leprosy (2 μm Eponembedded section stained with toluidine blue x 1000).
Electron microscopy of ultrathin sections cut from the same blocks confirmed that the structures were lymphatic vessels. In the lepromatous lesions, the lymphatic vessels usually had slit-like lumina. The lining cell cytoplasm was thin along the length of the vessel, while at the corners the cytoplasmic mass increased and numerous slender processes projected into the lumen. Mitochondria, lysosomes, lipid droplets, pinocytotic vesicles, and rough endoplasmic reticulum formed the bulk of the cytoplasm in these areas (Figs. 2 and 4). Large lipid droplets were present in many lymphatic lining cells (Fig. 2) and also occasionally in cells lying in the lumen of the lymphatic vessel. Lymphatic endothelial cells with intra-cytoplasmic bacilli were rare, seen in only one of the seven lepromatous biopsy specimens (Fig. 4). In another lepromatous patient, a histiocyte with a bacillus in its cytoplasm was seen in the lymphatic lumen (Fig. 3).
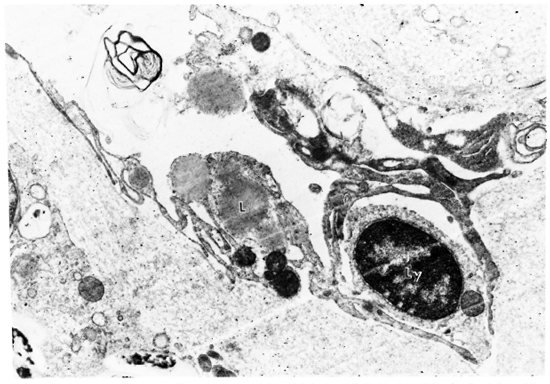
Fig. 2. Electron micrograph of lymphatic vessel from LL lesion. Lipid droplets (L), mitochondria and lysosome are seen in lymphatic endothelial cell cytoplasm; lymphocyte (Ly) traversing lymphatic wall; M. leprae in lower left corner (x 14,000).
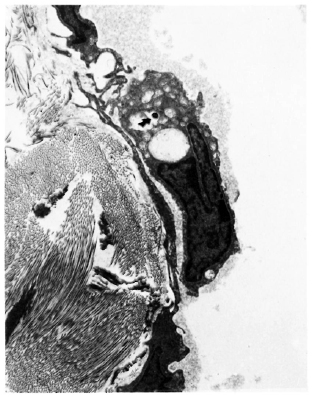
Fig. 3. Electron micrograph of a lymphatic vessel from a lesion of Iepromatous leprosy (LL). A macrophage containing a bacillus ( ) in its cytoplasm lies in the lymphatic lumen just over a point of overlap of two adjoining lymphatic endothelial cells. Elastic fibers lying in the immediate perilymphatic area are also seen (x9000).
) in its cytoplasm lies in the lymphatic lumen just over a point of overlap of two adjoining lymphatic endothelial cells. Elastic fibers lying in the immediate perilymphatic area are also seen (x9000).
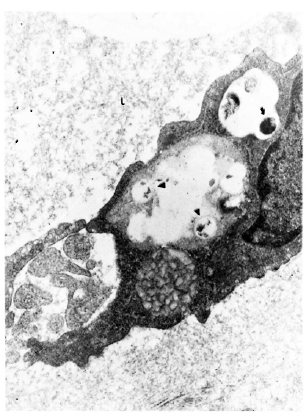
Fig. 4. Electron micrograph of a lymphatic lining cell in a lesion of Iepromatous leprosy (LL) showing a uniformly stained bacillus ( ) with an electron transparent zone around it lying in a cytoplasmic vacuole. Degenerated organisms (
) with an electron transparent zone around it lying in a cytoplasmic vacuole. Degenerated organisms (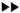 ) are also seen in the same cell. L = vessel lumen (x 23, 400).
) are also seen in the same cell. L = vessel lumen (x 23, 400).
Lymphocytes (Fig. 2) and histiocytes (Fig. 3) lying beneath the lining cell or in the intercellular junctional areas between two lymphatic endothelial cells were frequently observed in all specimens. Intact clastic fibers (Fig. 3) and anchoring filaments (Fig. 6) were identified along the abluminal aspect of the lining cells in all of the perigranulomatous and in some of the intragranulomatous lymphatics.
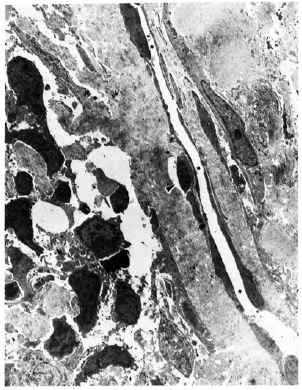
Fig. 5. Electron micrograph of a peri-granulomatous lymphatic (* marks the vessel lumen) from a lesion of borderline tuberculoid (BT) leprosy. Lining cell is indented by a lymphocyte (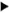 ) crossing the lymphatic wall. Cells comprising the granuloma are seen to the left and below the lymphatic vessel (x 2200).
) crossing the lymphatic wall. Cells comprising the granuloma are seen to the left and below the lymphatic vessel (x 2200).
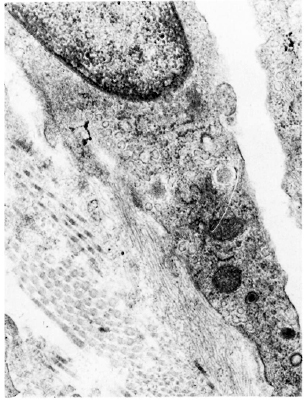
Fig. 6. Electron micrograph of a lymphatic lining cell from a lesion of lepromatous leprosy (LL). Cytoplasm contains numerous pinocytotic vesicles and abluminal surface of the cell shows a sheafofanchoring filaments in continuation with the plasma membrane (x 35, 600).
The tuberculoid lesions showed identifiable lymphatic vessels in the peri-granulomatous collagen only. Here, too, some vessels had slit-like lumina (Fig. 5), while others showed dilated and irregularly shaped lumina. The lymphatic endothelial cells in these lesions had fewer cytoplasmic organelles than those seen in lepromatous lesions. Lipid vacuoles were absent, and lysosomes were only seen in occasional cells. Lymphocytes traversing the lymphatic endothelium were frequently seen however. Bacilli were not found in the lymphatic endothelial cells in any of the eight tuberculoid cases studied.
DISCUSSION
This study demonstrates an actively functioning terminal lymphatic system in the cutaneous lesions of leprosy, as shown by activated and hypertrophied lymphatic endothelial cells, the presence of lipid vacuoles, and movement of inflammatory cells across the lymphatic wall.
The involvement of the lymphatic system in the clearance of lipidic material from the granuloma, especially in the bacilliferous lesion, is of importance in view of the fact that lipidic antigens of Mycobacterium leprae have been shown to be involved in immunostimulation (1). The movement of cells, both lymphocytes and histiocytes, through the lymphatic system is a regular function of the skin lymphatics. The large number of cells between lymphatic endothelial cells in both tuberculoid and Iepromatous cases further emphasizes the continued and increased functioning of the skin lymphatics in leprosy.
Surprisingly, only a few of the lymphatic endothelial cells in the Iepromatous lesions contained bacilli in their cytoplasm. Vascular endothelial cells, on the other hand, were much more heavily bacillated in all eight cases. Earlier reports (2,3,12,15) have shown consistent endothelial cell bacillation in blood capillaries in and around lesions. It seems that the blood vascular endothelial cell offers a better environment for bacillary growth than the lymphatic endothelial cell. The higher pO2 in the vascular endothelial cells may be a factor responsible for better growth of the microaerophilic M. leprae. Thus, compared to the blood vascular system, the lymphatic pathway seems a minor route for dissemination of M. leprae.
The few reports on the subject of skin lymphatics (7,8,11,13) indicate that elastic fibers and anchoring filaments are of primary importance in the functioning of the terminal lymphatic vessels. Both structures were seen in the lymphatics in the pcri-granulomatous areas and in a few of the intragranulomatous lymphatics in lepromatous lesions. The absence of intra-granulomatous lymphatics in tuberculoid lesions is of interest. It may be noted in this connection that in tuberculoid granulomas the reticulin framework is of an open type with large gaps toward the center of the granuloma, while in lepromatous lesions the reticulin fibers are more uniformly distributed throughout the lesion (6). The absence of the reticulin framework in the inner parts of the granuloma along with the destruction of the clastic fibers could lead to the disappearance of the intra-granulomatous lymphatics in the tuberculoid cases. The lymphatic function, however, continues to be maintained by the vessels at the granuloma edge which show movement of inflammatory cells through them.
Acknowledgments. AM was supported by an International Research Fellowship no. FO5 TWO 3595 from the Fogarty International Center, National Institutes of Health. Bethesda. Maryland, U.S.A.
REFERENCES
1. BRENNAN, P. J. The phthiocerol-containingsurface lipids of Mycobacterium leprae-a perspective of past and present work. Int. J. Lepr. 51(1983)387-396.
2. BURCHARD, G. and BIERTHER, M. An electron microscopic study of small cutaneous vessels in lepromatous leprosy. Int. J. Lepr. 53(1985)70-74.
3. CORUH, G. and MCDOUGALL, A. C. Untreated Iepromatous leprosy: histopathological findings in cutaneous blood vessels. Int. J. Lepr. 47(1979)500-511.
4. DESIKAN, K. V. and Jon, C. K. Leprous lymphadenitis. Int. J. Lepr. 34(1966)147-154.
5. FURNISS, A. L. Lymph glands in leprosy. Indian J. Med. Sci. 7(1953)475-481.
6. KHANOLKAR, V. R. The pathology of leprosy. In: Leprosy in Theory and Practice. Cochrane, R.G. and Davey. T.F., eds. Bristol: John Wright & Sons, Ltd., 1964, pp. 125-151.
7. LEAK, L. V. and BURKE, J. F. Fine structure of lymphatic capillaries and adjoining connective tissue area. Am. J. Anat. 118(1966)785-810.
8. LEAK, L. V. and BURKE. J. F. Studies on the permeability of lymphatic capillaries. J. Cell Biol. 50(1971)300-323.
9. LOWE. J. Tuberculoid changes in lymph nodes in leprosy. Int. J. Lepr. 7(1939)773-774.
10. MITSUDA, K. and OGAWA, M. A study of one hundred and fifty autopsies on cases of leprosy. Int. J. Lepr. 5(1937)53-60.
11. MORTIMER, P. S., CHERRY, G. W., JONES, R. L., BARNHILL, R. L. and RYAN, T. J. The importance of elastic fibres in skin lymphatics. Br. J. Dermatol. 108(1983)561-566.
12. MUKHERJEE, A. and MEYERS, W. M. Endothelial cell bacillation in lepromatous leprosy; a case report. Lepr. Rev. 58(1987)419-424.
13. RYAN, T. J., MORTIMER, M. D. and JONES, R. L. Lymphatics of the skin; neglected but important. Int. J. Dermatol. 25(1986)411-419.
14. SHARMA, K. D. and SRIVASTAVA, J. B. Lymph nodes in leprosy. Int. J. Lepr. 26(1958)41-50.
15. TURKEL, S. B., VAN HALE, H. M. and REA, T. H. Ultrastructure of the dermal microvasculature in leprosy. Int. J. Lepr. 50(1982)164-171.
1. M.D., Assistant Director, Institute of Pathology-I.C.M.R., P.O. Box 4909, Safdarjang Hospital, New Delhi 110029; India.
2. M.D., Dermatologist. Safdarjang Hospital. New Delhi 110029, India.
3. M.D., Ph.D., Chief, Division of Microbiology, Armed Forces Institute of Pathology, Washington, D.C. 20306, U.S.A.
Received for publication on 14 October 1988.
Accepted for publication on 8 December 1988.