- Volume 57 , Number 1
- Page: 20–3
Urinary excretion of renal brush-border enzymes in lepromatous leprosy-a preliminary investigation
ABSTRACT
Activities of the brush-border enzymes, alkaline phosphatase, maltase, leucine aminopeptidase, and gamma-glutamyl transpeptidase, were measured in urine samples of 25 lepromatous leprosy patients and an equal number of age-matched healthy controls. None of the patients were shown to be suffering f rom any other systematic disease. The enzymatic activities were shown to be significantly elevated in leprosy patients when compared to controls.RÉSUMÉ
Dans des échantillons d'urine provenant de 25 malades atteints de lèpre lépromateuse et d'un nombreégal de témoins en bonne santé assortis pour l'âge, on a mesuré l'activité d'une série d'enzymes de la bordure en brosse, la phosphatase alcaline, la maltase, la leucine aminopeptidase. et la gamma-glutamyl transpeptidase. On n'a mis en évidence aucune autre maladie systémique chez les malades. Les activités enzymatiques étaient significativement augmentées chez les malades de la lèpre, par comparaison avec les témoins.RESUMEN
Se midieron las actividades de las enzimas fosfatasa alcalina, maltasa, leucin aminopeptidasa y gamma-glutaminil transpeptidasa, en la orina dc 25 pacientes con lepra lepromatosa y en 25 controles sanos apareados por edad. Ninguno de los pacientes presentaba ninguna otra enfermedad sistémica al tiempo del estúdio. Se encontro que las actividades enzimáticas estuvieron significativamente incrementadas en el grupo de pacientes con lepra.Renal involvement has frequently been found to be associated with leprosy, often resulting in death (5, 7). A wide variety of histopathological and immunological lesions have been reported from different geographic areas (3, 6). Amyloidosis has been reported as one of the renal complications of leprosy by several workers(1, 14, 21, 22). It is well known that secondary changes occur in the renal tubules as a result of amyloidosis. Studies conducted by Gupta, et al. (11) Grover, et al. (10), and Date, et al. (5) show 14.4%, 5.6%, and 7.5% of amyloidosis in lepromatous leprosy, respectively. Phadnis, et al. (19) observed interstitial nephritis with varying amounts of tubular degeneration in 10 out of 28 biopsies. Mittal, et al. (16) detected tubular necrosis in three cases of lepromatous leprosy, with proteinaceous casts in two of them. One report of acute tubular necrosis has also been made by one of our authors (SK) in a previous study (23). Acute tubular necrosis has recently been reported in eight cases of leprosy by Date, et al. (5). Renal biopsy is a hazardous investigation. Moreover, there is a high likelihood that the site involved may be missed altogether in a renal biopsy because of the patchy nature of the pathology.
The damaged basement membrane sheds the brush-border enzymes in diseases which involve the renal parenchyma, and these enzymes are ultimately excreted in the urine. Therefore, the presence of these enzymes in the urine may reflect the extent of renal damage. To the best of our knowledge, impairment of renal function at the enzymatic level has not been studied in leprosy patients. This report is the result of a preliminary study conducted on urine specimens obtained from lepromatous leprosy patients.
MATERIALS AND METHODS
Patients. Twenty-five patients with lepromatous leprosy (classified according to Ridley and Jopling 20) from the leprosy clinic of the Nehru Hospital attached to the Postgraduate Institute of Medical Education and Research, Chandigarh, India, were studied. All patients were freshly diagnosed, untreated for leprosy, had not taken any other drugs during the past 6 months, and none had any other disease which could affect the kidneys. A 24-hr urine sample was collected in a sterilized container with sodium azide (1 g/1) to prevent contamination. The collections were done after hospitalization of the patients, and the samples were kept refrigerated (4ºC) and assayed in an air-conditioned room (cold room). The samples were assayed within 3 months of collection. The samples were centrifuged at 15,000 rpm and dialyzed against distilled water for 2 hr before the estimations of the enzymes were undertaken.
Controls. Twenty-five age-, sex-, and socioeconomic status-matched healthy individuals served as controls.
Estimation of brush-border enzymes. The enzymes studied were: a) alkaline phosphatase (Alph) (2); b) gamma-glutamyl transpeptidase (GGT) (18); c) leucine aminopep-tidase (LAP) (9); d) maltase (M) (4). Each enzyme was assayed in triplicate. The substrates used (Boehringer, Mannheim, West Germany) were: a) p-nitrophenyl phosphate in 0.5 M glycine buffer for Alph; b) gammaglutamyl p-nitroaniline in Tris-HC1 buffer,pH 7.0, for GGT; c) L-leucine-p-nitroanilide for LAP; and d) maltose for M. The enzymatic activities were estimated spectrophotometrically (LKB-Biochrom Ultraspec 4050) as determined by the colored products formed. The 24-hr urinary protein was also estimated by the method of Lowry, et al. (15)
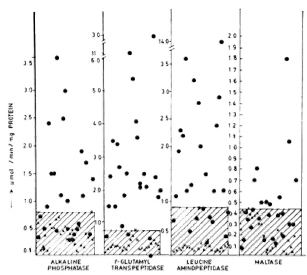
The Figure. Enzymatic activities of renal brush-border enzymes of control and test samples expressed as µmol/min/mg protein. The highest value of the control sample has been taken as the cut-off point [x = control samples;  = test (lepromatous leprosy) samples].
= test (lepromatous leprosy) samples].
RESULTS
The activities of the different renal brushborder enzymes estimated are shown as µmol/min/mg protein in The Figure. The highest value obtained for the control subjects in every estimation has been taken as the cut-offpoint for better comparison, and values beyond this were taken as positive. A significant increase (p < 0.01) was seen in the activities of alkaline phosphatase, leucine aminopeptidase, and gamma-glutamyl transpeptidase in the leprosy group compared to controls. The increase in excretion of maltase was statistically even more pronounced (p < 0.001) in the leprosy group compared to controls. The mean values of the 24-hr urinary protein estimated (0.26 ± 0.11 mg/ml with a range of 0.11-0.56 mg/ ml) in leprosy patients were increased (p < 0.001) when compared to those of the control samples (0.11 ± 0.03 mg/ml with a range of 0.05-0.2 mg/ml).
DISCUSSION
A high incidence of renal abnormalities is reported to occur in lepromatous leprosy (7, 12), indicating that the kidney is one of the target organs involved and that it is rendered more susceptible to systemic infections during the course of the disease. The histopathological aspects of renal involvement in leprosy do not, by themselves, explain the complete renal pathogenesis.
In the present investigation, 11-15 out of 25 lepromatous leprosy patients studied showed increased quantities of kidney-associated enzymes in their urine. No other evidence of renal disease was seen. Hence, it may be presumed that the patients in whom high levels of urinary enzymes were detected are in the preclinical stage of tubular damage probably related to their lepromatous leprosy. Gamma-glutamyl transpeptidase, one of the most important renal brush-border enzymes, was excreted in almost every patient. The effect of treatment on the rate of excretion of these enzymes might be useful in a follow-up study.
Studies of the urinary excretion of brushborder membrane enzymes has been carried out both in animals and in man (17, 24). Studies in lithium-administered rats have indicated that the urine enzyme assay is a valuable tool for detecting renal damage (8). Jung, et al. (13) have studied the enzymatic activities in patients with chronic glomerulonephritis and chronic pyelonephritis and found that the diagnostic importance obtained from an estimation of total activity can be further improved by separating the enzymes into soluble and particulate forms.
Systemic infections such as leprosy can cause necrosis of the proximal tubules, resulting in shedding of the enzymes into the urine. The detection of enzymuria may have many implications. It might be helpful in follow-up studies of patients under treatment or, alternatively, to check for relapses because the release of kidney-associated enzymes can well be a marker of ongoing active damage. It is possible that the tubular damage picked up from the shedding of the epithelial membrane (and consequently the enzymes) might be occurring prior to the histopathological damage, and thus enzymuria might be useful as an early marker of renal damage.
REFERENCES
1. Atta, A. G., Fleury, R. N., Maringoni, R. L., Trindade, A. S., Jr., Rufino, C. B. F. and Filho, B. S. Renal amyloidosis in leprosy. Int. J. Lepr. 45(1977)158-166.
2. Bergmeyer, H. U., ed. Methods of Enzymatic Analysis. New York: Academic Press, 1963, p. 783.
3. Chugh, K. S., Damle, P. B., Kaur, S., Sharma, B. K., Kumar, B., Sakhuja, V., Nath, I. V. and Datta , B. N. Renal lesions in leprosy amongst north Indian patients. Postgrad. Med. J. 59(1983)707-711.
4. Dahlqvist, A. Method for assay of intestinal disaccharidasc. Anal. Biochem. 7(1964)18-25.
5. Date, A., Harihar, S. and Jeyavarthini, S. E. Renal lesions and other major findings in necropsies of 133 patients with leprosy. Int. J. Lepr. 53(1985)455-460.
6. Date, A. and Johny, K. V. Glomerular subepithelial deposits in lepromatous leprosy. Am. J. Trop. Med. Hyg. 24(1975)853-856.
7. Desikan, K. V. and Jon, C. K. A review of postmortem findings in 37 cases of leprosy. Int. J. Lepr. 36(1968)32-44.
8. Emanuelli, G., Anfossi, G., Calcamuggi, G., Marcarino, C, Ottone, G. and Dughera, L. Urinary enzyme excretion in acute and subacute experimental lithium administration. Enzyme 34(1985)177-185.
9. Goldbarg, J. A. and Rutenberg, A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer 11(1958)283-291.
10. Grover, S., Bobhate, S. K. and Chaubey, B. S. Renal abnormality in leprosy. Lepr. India 55(1983)286-291.
11. Gupta. S. C, Bajaj, A. K., Govil, D. C, Sinha, S. N. and Kumar . R. A study of percutaneous renal biopsy in lepromatous leprosy. Lepr. India 53(1981)179-184.
12. Gupta, J. C, Diwakar, R., Singh, S., Gupta, D.K. and Panda , P. K. A histopathologic study of renal biopsies in 50 cases of leprosy. Int. J. Lepr. 45(1977)167-170.
13. Jung, K. and Sehulize, G. Diuresis dependent excretion of multiple forms of renal brushborder enzymes in urine. Clin. Chim. Acta 156(1986)77-84.
14. Krishnamurthy, S. and Job, C. K. Secondary amyloidosis in leprosy. Int. J. Lepr. 34(1966)155-158.
15. Lowry, D. H., Rosenbrough, N. J., Fars, A. L. and Randall . R. J. Protein measurement with folin-phenol reagent. J. Biol. Chcm. 193(1951)265-275.
16. Mittal, M. M., Maheshwari, H. B. and Kumar, S. Renal lesions in leprosy. Arch. Pathol. 93(1972)8-12.
17. Mohndorf, A. W., Breier, J., Hendus, J., Seher- berich, J. E., Nakenrodt, G., Shah, P. M., Stille, W. and Sehoeppe , W. Effect of aminoglycosides on proximal tubular membranes of the human kidney. Eur. J. Clin. Pharmacol. 13(1978)133- 142.
18. Naftalin, L., Sexton, M., Whitaker, J. F., et at. A routine procedure for estimating scrum gammaglutamyltranspeptidase activity. Clin. Chim. Acta 26(1969)293-296.
19. Phadnis, M. C, Mehta, M. C, Bharaswadker, M. S., Kolhatkar, M. K. and Bulakh, P. M. Study of renal changes in leprosy. Int. J. Lepr. 50(1982)143-147.
20. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
21. Satyanarayana, B. V. Raju, P. S., Kumari. K. R. and Reddy, C. R. R. M. Amyloidosis in leprosy. Int. J. Lepr. 40(1972)278-280.
22. Shuttleworth , J. S. and Ross, Sr. H. Secondary chem. Pharmacol. 23(1974)65-73. amyloidosis in leprosy. Ann. Intern. Med. 45(1956)23-28.
23. Singhal, P. C, Chugh, K. S., Kaur, S. and Malik , A. K. Acute renal failure in leprosy. Int. J. Lepr. 45(1977)171-174.
24. Wright, P. J. and Plummer, D. I. The use of urinary eniyme measurements to deteet renal damage eaused by nephrotoxie compounds. Biochem. Pharmacol. 23(1974)65-73.
1. M.Se., Ph.D. Resident, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
2. Ph.D., Tutor, Department of Dermatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
3. Ph.D., Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
4. M.D., F.A.M.S., Professor and Head, Department of Experimental Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
5. M.D., M.A.M.S., Professor and Head, Department of Dermatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
Reprint requests to Professor S. Kaur.
Received for publication on 5 February 1988.
Accepted for publication in revised form on 28 October 1988.