- Volume 57 , Number 1
- Page: 24–32
Appraisal of two Mycobacterium leprae-specific serological assays for monitoring chemotherapy in lepromatous (LL/BL) leprosy patients
ABSTRACT
Two of the Mycobacterium leprae-specific assays -a serum antibody competition (for an epitope on 35-kDa protein) test (SACT) and an enzyme-linked immunosorbent assay (ELISA) for the disaccharide epitope of phenolic glycolipid-I (PGDS) - were comparatively evaluated as tools for monitoring chemotherapy in 125 lepromatous leprosy (LL/BL) patients. An adaptation of the SACT f rom a radioimmunoassay (RIA) to an ELISA procedure is also described. A moderate but statistically significant correlation was observed between the assays, although SACT appeared to be the more sensitive of the two. Levels of antibodies correlated better with the bacterial index (BI) than with the duration of treatment. However, wide individual variations in antibody levels (for a specific duration of treatment or BI) were seen in treated as well as untreated patients. Anti-PGDS antibody response of the IgG type was poorer than that of the IgM type and, apparently, it did not have a bearing on either treatment duration or the BI. Further studies will be needed to clarify whether the treated patients showing a negative (or low) BI and high antibody levels were harboring hidden foci of active infection, and whether treatment could safely be terminated in patients showing low values for both BI and antibody.RÉSUMÉ
Chez 125 malades atteints de lèpre lépromateuse (LL/BL), on a procédé à une évaluation comparative de deux épreuves spécifiques pour Mycobacterium leprae, à savoir une épreuve de compétition spécifique des anticorps pour un epitope situé sur la protéine 35-kDa (SACT), et une épreuve ELISA dirigée contre l'épitope disaccharide de l'antigène phéno-glycolipidique-I (PGDS). Cette étude a été menée en vue de comparer la valeur de ces épreuves pour la surveillance de la chimiothérapie. On décrit également l'adaptation de l'épreuve SACT à partir d'un essai radio-immunologique (RIA) pour la transformer en une procédure ELISA. Une corrélation modérée mais statistiquement significative a été observée entre ces épreuves, encore que l'épreuve SACT apparaisec être la plus sensible des deux. Les taux d'anticorps présentaient une meilleure corrélation avec l'index bactériologique (BI) qu'avec la durée du traitement. Néanmoins on a constaté de grandes variations individuelles dans les taux d'anticorps (ceci pour une durée spécifique du traitement ou un index bactériologique donné), tant chez les malades traités que chez ceux qui ne l'étaient pas. La réponse en anticorps anti-PGDS du type IgG était plus faible que celle constatée pour les anticorps de type IgM. Apparemment, ces réponses n'étaient pas en relation avec la durée du traitement ou l'index bactériologique. Des études complémentaires sont requises en vue de savoir si les malades traités qui présentent à la fois un index bactériologique négatif ou faible et des taux élevés d'anticorps, sont porteurs de foyers cachés d'infection active, et si le traitement peut être terminé sans risques chez les malades qui présentent à la fois des valeurs basses pour l'index bactériologique et pour les anticorps.RESUMEN
Se evaluaron en forma comparativa dos ensayos específicos para Mycobacterium leprae con el fin de utilizarlos en el seguimiento de 125 pacientes lepromatosos (LL/BL) sujetos a quimioterapia. Los ensayos fueron la prueba de competencia del anticuerpo sérico (por un epitope sobre la proteína 35-kDa) (SACT) y un inmunoensayo enzimático (ELISA) para el epitope disacárido del glicolípido fenólico-I (PGDS). También se describe una adaptación de un radioinmunoensayo para SACT a un procedimento de ELISA. Se observó una correlación moderada pero estadísticamente significativa entre los ensayos, aunque SACT pareció ser el más sensible. Los niveles de anticuerpos correlacionaron mejor con el índice bacteriano (BI) que con la duración del tratamiento. Sin embargo, se observaron amplias variaciones individuales en los niveles de anticuerpo en los sujetos tratados y en los no tratados (a duración de tratamiento o BI específicos). La respuesta en anticuerpo anti-PGDS de la clase IgG fue más pobre que la respuesta del tipo IgM y, aparentemente, esto fue independiente del tiempo de tratamiento o del BI. Se requiere más trabajo para aclarar si los pacientes tratados con un BI negativo (o bajo) y altos niveles de anticuerpo, son portadores de focos ocultos de infección activa, y si el tratamiento puede terminarse con seguridad en los pacientes que muestran valores bajos tanto del BI como de anticuerpos.In the absence of primary prevention, the early diagnosis and efficient treatment of leprosy continue to be the basis of all leprosy control programs (19). The effect of antileprosy treatment is monitored by periodic assessment of the degree of bacillary load (Mycobacterium leprae) in the patient's skin as determined by smear microscopy and the bacterial index (BI) (17). In special situations, assessment of bacterial viability by its ability to multiply is made by using mouse foot pad inoculation studies (22). These same methods are also applied to detect failure of a treatment resulting from such cases as bacterial persistence, drug resistance, and relapse (6). However, both methods have their limitations. Skin-smear examination and B1 determinations are prone to sampling errors. Further, they do not give an idea about the quantum of infection in different areas of the skin and in other tissues of the body, such as the nerves (27). The mouse foot pad inoculation studies require animal house facilities and expertise. Moreover, the normal mouse docs not allow the growth of small numbers of viable M. leprae present in a large pool of dead bacilli (5). In view of these constraints, several attempts are currently being made to develop alternative techniques for monitoring active infection in leprosy patients (4, 9, 13, 15, 21).
Assays for M. leprae-specific antibodies(20) in relation to the BI have also been used for monitoring treatment on the basis that antibody titers would reflect the antigen load in the whole body and, therefore, could serve as a good indicator of regressing, sustaining, or progressing infection in patients. In the present study, we have applied serological assays for M. leprae-specifc antibodies to the 35-kDa protein (24, 25) and phenolic glycolipid-I (PGL-I) (2, 3) antigens in a comparative manner. Previous studies in this direction have focused attention on the latter assay (for anti-PGL-I antibodies) using small-sized samples, mixing patients of different types of leprosy together, and testing under varying experimental conditions (1, 11, 12). Presently, we have confined our investigations to lepromatous patients, since they require more judicious and prolonged treatment compared to tuberculoid patients. The adaptation of the serum antibody competition (for 35-kDa protein antigen) test (SACT) as an enzyme-linked immunosorbent (ELISA) procedure instead of a radioimmunoassay (RIA) is also described.
MATERIALS AND METHODS
Study subjects. One hundred-twenty-five patients with lepromatous (LL) or borderline lepromatous (BL) leprosy (18) attending the clinics of the Central JALMA Institute for Leprosy, Agra, India, were included in the study. Seventeen were untreated and 108 were receiving dapsone monotherapy or multidrug therapy (MDT) (dapsone, clofazimine, rifampin) for periods up to 200 months, as obtained from case records. Most of the patients on MDT had received dapsone monotherapy in the beginning. Sera obtained from 62 healthy subjects (Indian and European) and 20 pulmonary tuberculosis patients served as controls for standardization of the serological tests.
Bacterial index (BI).Four slit-skin smears prepared from each patient (from both earlobes and at least two active sites, generally on the arm and back) were used for determination of the average BI, according to Ridley's logarithmic scale (17).
ELISA for anti-PGL-I disaccharide (PGDS) antibodies. The method described by Cho, et al. (2, 3) was followed with minor modifications. Briefly, ELISA plates (Immunoplate II; Nunc, Denmark) were coated (50 µl/well, 37ºC, overnight, in humidity) with either carbonate buffer (pH 9.5) or 1:10,000 dilution (in carbonate buffer) of phenolic glycolipid-I disaccharide-bovine serum albumin (PGDS-BSA) conjugate (kindly supplied by Dr. Delphi Chatterjee, Department of Microbiology, Colorado State University, Colorado, U.S.A.; prepared and lyophilized at a ratio of 100 µg PGDS to 450 µg BSA per 0.5 ml). The plates were washed with phosphate buffered saline (pH 7.4) containing 0.05% Tween 20 (PBST) and blocked (100 µ/well, 2 hr, 37ºC) with 1% BSA in PBST (BSA-PBST). Serum dilutions (1:300 in BSA-PBST) were incubated in triplicate wells (50 µ/well, 60 min, 37ºC) coated with either buffer or antigen. After washing with PBST, the plates were incubated ( 50 µ/well, 60 min, 37ºC) with 1:1000 diluted (in BSA-PBST) peroxidaselabeled anti-human IgM or anti-human IgG antibody (Cappel). Color was developed by incubating the plates with 0-phenylenediaminc (Sigma) substrate solution (50 µ/well, 20 min, 37ºC). After stopping the reaction with 7% H2SO4 (µ/well), the optical densities (ODs) were read at 492 nm using an ELISA reader (Titretek Multiskan Plus, Flow Laboratories). For each serum, the mean OD of the buffer-coated wells was subtracted from the mean OD of the antigen-coated wells.
On the basis of the results obtained with nonleprosy controls (mean ± 2 S.D.), a serum (at 1:300 dilution) was regarded as PGDS-ELISA positive if it showed an OD of > 0.20 for IgM- and > 0.15 for IgG-type antibodies. A pooled LL serum was tested every time the PGDS-ELISA was done to assess the extent of interassay variation. In the final analysis (after six separate assays), it showed an OD of 1.13 ± 0.13 (mean ± S.D.) for IgM and 0.05 ± 0.05 for IgG antibodies.
Serum antibody competition test (SACT). Initially, a SACT was performed using the inhibition RIA procedure which has already been described (24, 25). Later, it was successfully converted to an inhibition ELISA. Optimal assay conditions and the results obtained were identical to those of SACT-RIA (25). Briefly, each ELISA plate (Immunoplate I or II; Nunc, Denmark) was coated with either PBS (50 µ/well in the first row, 4ºC, overnight, in a humid box) or soluble antigen (50 µ/well protein/ml in phosphatebuffered saline (PBS), 50 µ/well, in rows 2, 12) of M. leprae (armadillo derived, a kind gift from IMMLEP, supplied by Dr. R. J. W. Rees, Clinical Research Center, Harrow, Middlesex, U.K.). The antigen was collected (for repeated use), and the plates were washed twice with PBST before blocking with 1% BSA in PBST (100 µ/well, 2 hr, room temperature). Duplicate wells in rows 3-12 (antigen coated) of a plate were incubated (60 min, 37ºC) with serial dilutions (1:5 to 1:10,000 or more in BSA-PBST) of each serum (25 µ/well). The first row (PBS coated) and second row (antigen coated) were incubated with BSA-PBST (µ/well) alone (instead of serum dilutions) so as to yield 0% (reagent blank) and 100% (maximum) bindings (in terms of OD), respectively. The plate was further incubated (2 hr, 37ºC) after adding 1:500 dilution (in BSA-PBST, 25 µ/well) of peroxidase -conjugated monoclonal antibody, ML04 (kindly supplied by Dr. J. Ivanyi, MRC Tuberculosis and Related Infections Unit, Hammersmith Hospital, London, U.K.) in all wells. The remaining steps (washing, color development, reading) were the same as for the PGDS-ELISA described above.
The mean 0% (reagent blank) OD was subtracted from each of the test OD values. The OD for 100% (maximum) binding varied from plate to plate and day to day (range 0.7 to 1.0; median 0.8). Probably the repeated use of coating antigen was mainly responsible for the variation. Relative percent bindings were calculated using 100% OD value for that plate only and plotted against the corresponding dilutions of a serum (25). The dilution which would cause 50% inhibition of the maximum binding (100% OD value) of peroxidase-ML04 (100% OD value) is referred to as the ID50 titer of a serum.
On the basis of results obtained with nonleprosy controls in this study and in the previous studies (23-25), a serum with an ID50 titer of > 5 (> 1:5) was regarded as SACT positive. The reference LL serum pool, tested on six occasions, showed an ID50 value (mean ± S.D.) of 804 ± 100.
Statistical analysis. Analysis was done by computer using the BMDP statistical packages (BMDP Statistical Software, Inc., Los Angeles, California, U.S.A.; 1987 program version).
RESULTS
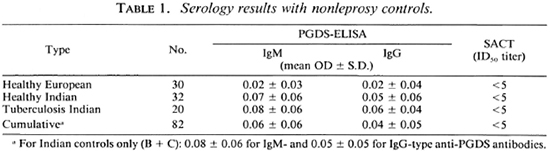
Serological assays. The assay results with healthy controls and tuberculosis patients are shown in Table 1. For PGDS-ELISA, the mean OD ± 2 S.D. (95% confidence limits) of Indian controls only was regarded as the cut-off point (0.20 for IgM and 0.15 for IgG antibodies; Table 1). None of the control sera tested for SACT in this study (Table 1) and in the previous studies (23-25) was able to show an ID50 titer of > 5 (1:5, the tested minimum dilution). Thus, any serum with an ID50 value of > 5 (> 1:5) was regarded as SACT positive.
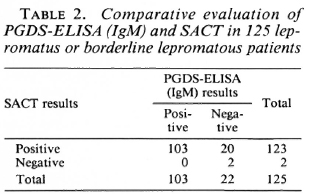
The predominant antibody response to PGDS was of the IgM type (compared to the IgG type of response). Of the 125 lepromatous patients studied, the PGDS-ELISA (for IgM class antibodies) was found positive in 103 patients (82.4%). On the other hand, the SACT gave positive results in 123 patients (98.4%). As may be seen from Table 2, none of the patients positive for PGDS-ELISA was negative for SACT, while 20 of the 123 patients positive for SACT gave negative results by PGDS-ELISA. Thus, the SACT was found to be the more sensitive (d = 16%, S.E. = 3.58, p < 0.001) of the two assays.
A correlation was sought between the PGDS-ELISA and SACT (Fig. 1). Although there were patients showing high SACT titers and low IgM levels (and vice versa), a moderately positive and statistically significant correlation was noted between the results of the two assays (r = 0.463, p < 0.001).
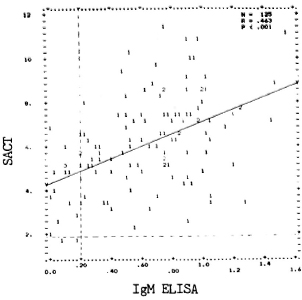
Fig. 1. Correlation between PGDS-ELISA andSACT values in 125 LL/BL patients. Optical densities(OD.2) for IgM antibodies are represented on "x" axisand "y" axis indicates natural logarithms (loge10 =2.30259, loge100 = 4.60517, and so on) of ID50 values. Numbers denote the number ofidentical results. Upperlimits of PGDS-ELISA and SACT values in nonleprosycontrols are indicated, respectively, by vertical andhorilontal dotted lines. Regression line (y-y) is alsodrawn.
Duration of treatment and BI. For any specific duration of treatment, the average BI of these patients showed a wide range (Fig. 2). A few patients had attained skinsmear negativity as early as 18 months after treatment; a few others have remained positive even after 10 years of treatment. However, a moderate but significant negative correlation (r = -0.458; p < 0.001) was observed between the BI and the duration of treatment in this group of patients.
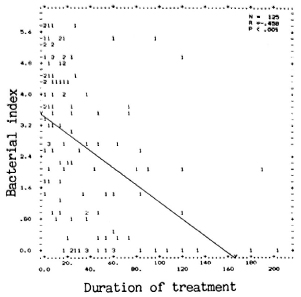
Fig. 2. Correlation between III and treatment du-ration in months. (Other details are as in Fig. 1.)
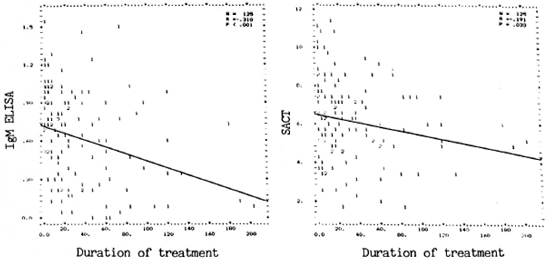
Fig. 3. Correlation between antibody assay results and treatment duration in months. Left panei showscorrelation between PGDS-ELISA (IgM) and duration; right panei shows correlation between SACT and duration.(Other details are as in Fig. 1.)
Duration of treatment and antibody levels. As with the BI, wide variations were also seen in levels of both the assays (SACT and PGDS-ELISA) for IgM antibodies in untreated and treated patients (Fig. 3). Although all of the untreated patients (treatment duration = 0) were positive for both assays, some of the treated patients eventually showed negativity, particularly for anti-PGDS antibodies. A weak but significant negative correlation (r = -0.31, p < 0.001) was observed between duration of treatment and anti-PGDS antibody, but not between duration of treatment and SACT.
BI and antibody levels. Individual antibody levels varied widely for any value of the BI, including when the BI was zero (Fig. 4). Nevertheless, a moderately positive significant correlation was noted between the BI and the SACT titers (r = 0.458, p < 0.001) as well as between the BI and PGDS antibody (IgM) levels (r = 0.43, p < 0.001). It will be seen (Fig. 4) that about 10% of patients with a positive BI (up to 4.75) were negative by PGDS-ELISA (OD < 0.2); whereas only one (1%) Bl-positive case (BI = 1.5) was negative for SACT (ID5 0 < 5).
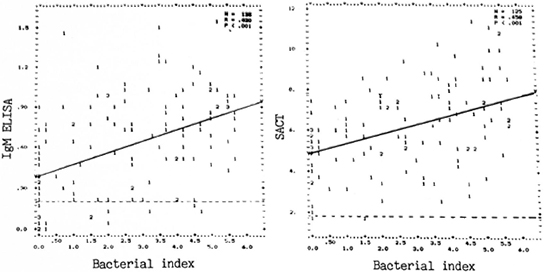
Fig. 4. Correlation between antibody assay results (left panei = PGDS-ELISA, IgM; right panei = SACT)and Bi. (Other details are as in Fig. 1.)
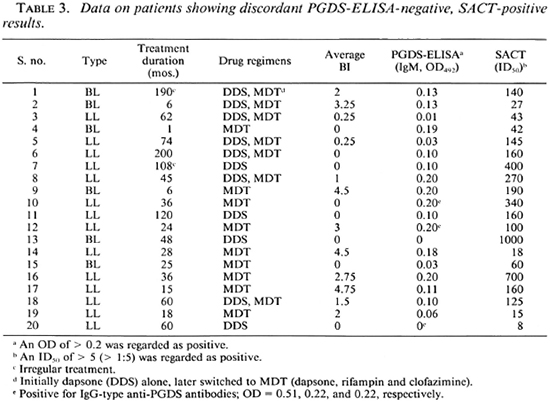
Analysis of discordance between the two tests. Individual data on the 20 patients who were positive for SACT (ID5( 1 > 5) but negative for PGDS-ELISA (IgM, OD < 0.2) are compiled in Table 3. Apparently the type of leprosy (LL or BL), the duration of treatment (1 to 200 months), or the drugs administered (dapsone alone or MDT) were not responsible for this discordance. Twelve (60%) of the discordant sera were obtained from Bl-positive patients (BI range 0.25 to 4.75). These data once again suggest the greater sensitivity of the SACT. However, the SACT results in this group of patients were only slightly to moderately positive (ID50 range 8 to 1000), considering that the upper limit of ID50 titers in this study was 98,000. Interestingly, 3 of these 20 patients showed positivity of the IgG type of anti-PGDS antibodies despite being negative for the IgM type of corresponding antibodies.
Role of anti-PGDS antibody (IgG). The assay for this type of antibody was not useful in the assessment of treatment since even a majority of the untreated patients (with high BI) showed negativity for this assay. No significant correlations were seen between antibody levels and the BI or duration of treatment (Fig. 5).
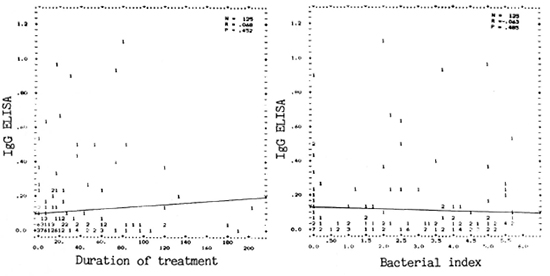
Fig. 5. Correlation between PGDS-ELISA (1gG) and duration of treatment (left panei) or BI (right panei).
DISCUSSION
It is generally hoped that treatment failures (bacillary persistence, drug resistance, and relapse) commonly encountered during dapsone monotherapy (7) will be minimized with the advent of MDT for leprosy (28). However, bacillary resistance to each of the drugs used for the combinations has been observed, and the problem could be further aggravated by irregular treatment (7, 10). Thus, a need has been felt to monitor the drug trials carefully with the help of new, more sensitive tools. This need is greatest in patients who are primarily resistant to one of the drugs (6).
The present study was aimed at assessing the value of two of the M. leprae-specific serological assays, SACT and PG-ELISA, as a tool for monitoring chemotherapy in leprosy patients. The present and previous data(1-3. 11, 12, 14, 20, 23-25) have repeatedly indicated that both of the serological assays are M. leprae specific with respect to the practiced criteria for seropositivity. The phenomenon of steric hindrance (apparent inhibition of binding of specific antibody due to the binding of other antibodies at adjacent antigenic sites) might be operational in the case of SACT, but only to the extent that all of the nonleprosy control sera (present study and 23-25) as well as nearly 50% of the tuberculoid leprosy sera (25) show an ID50 titer of < 5 (considered as assay negative). On the other hand, as apparent from the present data, the upper limit of the ID50 titer for a SACT-positive serum could be as high as 98,000.
The sensitivity of the SACT assay appeared to be greater than the PGDS-ELISA, considering that none of the PGDS-ELISApositive patients were negative for SACT; whereas 20 (16%) of SACT-positive patients (12 of whom had a positive BI as well) were negative for the PGDS-ELISA (IgM). An analysis of the data on leprosy type, duration, kind of treatment, and BI did not reveal the reason for this discordance. Nevertheless, a moderately positive and statistically significant correlation was noted between the results of the two assays.
Despite a moderately negative correlation between the BI and duration of treatment, the scatter of the BI values in patients who were either untreated or treated for specific durations indicates wide individual variations within a class of leprosy patients. This heterogeneity, also evident from the antibody levels, should be an important consideration while analyzing the data. The pattern of antibody scatter did not vary even when patients receiving either dapsone alone or polychemotherapy were grouped apart (data not shown).
A slightly better correlation was observed between the BI and the levels of either of the antibodies. Such a correlation has been observed by some investigators using the PG-ELISA (1, 11) but not by others (12, 26). Many skin-smear-positive patients (BI up to 4.75) receiving treatment showed negativity for the PGDS-ELISA (OD < 0.2) but not for the SACT (ID5 0 < 5), which once again indicates that the SACT was the more sensitive of the two assays (23). It has been proposed to terminate treatment in leprosy patients much before the attainment of skinsmear negativity for acid-fast bacilli, considering that the remaining "dead" bacilli would be eliminated by the immune system of the host 8). It would be interesting to know whether the patients with positive BIs but negative (or low) antibody levels are harboring dead bacilli or, in other words, whether these assays can differentiate between bacterial persistence and antigenic persistence.
Other reasons for the observed scatter in antibody levels at any specific positive BI value could be: a) M. leprae residing in the deeper tissues of the body might contribute to an enhanced antigenic load, which is overlooked by estimating the BI alone, in some patients (16, 27). This view could explain the fact that many patients showed raised antibody levels even at zero BI. Such hidden foci of infection persisting in the body are considered to be responsible for drug resistance and relapses (6). Thus, it would be important to monitor the patients who show high antibody levels despite a low or zero BI after stopping treatment. Their follow-up would also help in establishing that the treatment could safely be stopped in patients showing a low BI as well as low antibody levels, b) Individual differences in the B-cell response to antigens might also be responsible for the observed scatter of antibody levels at a particular BI value. Better understanding of this and other aspects would require studying each patient longitudinally. Such attempts have been made, although with limitations, by some workers(1, 14)
One of our previous studies (using a smaller sample size) had indicated that levels of the IgG class of anti-PGL-I antibodies might increase with a decrease in the antigenic load following treatment (23). In the present study also, 3 out of 20 PGDS-ELISA (IgM) negative patients were positive for IgG class antibodies (Table 3). However, when all of the results were analyzed together, we did not observe any correlation between IgG and the BI or duration of treatment. This observation is in agreement with a recent report (12).
Acknowledgments. We thank Dr. R. J. W. Rees, Clinical Rese are h Centre, Harrow, Middlesex, U.K., for the supply of M. leprae antigens; Dr. J. Ivanyi, MRC Tuberculosis and Related Infections Unit, Hammersmith Hospital. London, U.K., for the supply of peroxidase-conjugated ML04; and Dr. Delphi Chatterjee, Colorado State University, Colorado, U.S.A., for the PGDS-BSA conjugate. Healthy European control sera were kindly provided by Dr. H. D. Engers, Secretary. IMMLEP, World Health Organization, Geneva, Switzerland. We also thank Mr. H. O. Agarwal for photography and Mr. Anil Kumar Chopra for secretarial help. Technical assistance was provided by Mr. P. N. Sharma, Mr. R. Dayal, and Mr. M. Alam. The manuscript was kindly reviewed by Dr. H. Srinivasan, Director, Central JALMA Institute for Leprosy, Agra.
The work was partly supported by ad hoc grants from the Indian Council of Medical Rese are h. Ms. A. McEntegart was supported by a grant from LEPRA.
REFERENCES
1. Bach, M-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid-I and to whole M. leprae in leprosy patients: evolution during therapy. Int. J. Lcpr. 54(1986)256-267.
2. Cho, S.-N., Fujiwara, T., Hunter. S. W., Rea, T. H., Gelber, R. H. and Brennan, P. J. Use of an artificial antigen containing the 3, 6-di-Omethyl-β-D-glucopyranosyl epitope for the serodiagnosis of leprosy. J. Infect. Dis. 150( 1984)311-322.
3. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan. P. J. Serological specificity of phenolic glycolipid-I for M. leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(183)1077-1083.
4. Dhople, A. M. and Hanks, J. II. Adenosine triphosphate content in M. leprae; a brief communication. Int. .1. Lepr. 49(1981)57-59.
5. Gelber, R. H., Humphres, R. C. and Fieldsteel, A. H. Superiority of the neonatally thymectomized Lewis rat(NTLR)to monitor a clinical trial in lepromatous leprosy of the two regimens of rifampin and dapsone. Int. J. Lepr. 54(1986)273-283.
6. Grosset, J. H. Recent developments in the field of multidrug therapy and future rese are h in chemotherapy of leprosy. Lepr. Rev. 57 Suppl. 3(1986)223-234.
7. Ji, B. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
8. Jopling, W. H. A report on two follow-up investigations of the Malta project, 1983 and 1986. Lepr. Rev. 57 Suppl. 3(1986)47-52.
9. Kvach, J. T., Munguia, G. and Strand, S. H. Staining tissue derived M. leprae with fluorescein diacetate and ethidium bromide. Int. J. Lepr. 52(1984)176-182.
10. Leiker, D. L., Dhople, A. M. and Freerksen, E. Final bulletin. Lepr. Rev. 57 Suppl. 3(1986)274-277.
11. Levis, W. R., Meeker, H. C, Schuller-Levis, G., Sersen, E. and Schwerer, 15. IgM and IgG antibodies to phenolic glycolipid-I from M. leprae in leprosy: insight into patient monitoring, ENL and bacillary persistence. J. Invest. Dermatol. 86(1986)529-534.
12. Lyons, N. F., Shannon, E. J., Ellis, 13. P. 13. and Naafs, 13. Association of IgG and IgM antibodies to phenolic glycolipid-I antigen of M. leprae with disease parameters in multibacillary leprosy patients. Lepr. Rev. 59(1988)45-52.
13. Mahadevan, P. R., Jagannathan, R., Bhagaria, A., Vejare, S. and Agarwal, S. Host-pathogen interaction -new in vitro drug test systems against Mycobacterium leprae- possibilities and limitations. Lepr. Rev. 57 Suppl. 3(1986)182-200.
14. Miller, R. A., Gorder, D. and Harnisch, P. Antibodies to phenolic glycolipid-I during long-term therapy: serial measurements in individual patients. Int. J. Lepr. 55(1987)633-636.
15. Mittal, A., Sathish, M., Seshadri, P. R. and Nath, I. Rapid radiolabelled microculture method that uses macrophages for in vitro evaluation of M. leprae viability and drug susceptibility. J.Clin. Microbiol. 17(1983)704-707.
16. Ramu, G. and Desikan, K. V. A study of scrotal biopsy in subsided cases of lepromatous leprosy. Lepr. India 51(1979)341-346.
17. Ridley. D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T.F., eds. Baltimore: Williams and Wilkins, 1964, pp. 620-622.
18. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34(1986)255-273.
19. Sansarricq, H. Leprosy in the world today. Lepr. Rev. 52 Suppl.(1981)15-31.
20. Serological tests for leprosy.(Editorial)Lancet 1(1986)533-535.
21. Seydel, U. and Lindner, 13. Single bacterial cell mass analysis: a rapid test method in leprosy therapy control. Lepr. Rev. 57 Suppl. 3(1986)163-170.
22. Shepard, C. C, Walker, L. L., van Landingham, R. M., and Redus, M. A. Kinetic testing of drugs against Mycobacterium leprae in mice. Am. J. Trop. Med. Hyg. 20(1971)616-620.
23. Sinha, S., Patil, S. A., Girdhar, 13. K., Ramu, G., Sengupta, U. and Rees, R. J. W. A comparative study of phenolic glycolipid ELISA(PG-ELISA)and serum antibody competition test(SACT)on a set of serum samples. In: Proceedings of the Indo-U.K. Symposium on Leprosy, Agra, April 7-10, 1986, Katoch, V. M., cd. Agra: Central JALMA Institute for Leprosy(ICMR), 1986, pp. 45-51.
24. Sinha, S., Sengupta, U.. Ramu, G. and Ivanyi, J. A serological test for leprosy based on competitive inhibition of monoclonal antibody binding to the MY2a determinant of M. leprae. Trans. R. Soc. Trop. Med. Hyg. 77(1983)869-871.
25. Sinha, S., Sengupta, U., Ramu, G. and Ivanyi, J. Serological survey of leprosy and control subjects by a monoclonal antibody based immunoassay. Int. J. Lepr. 53(1985)33-38.
26. Truman, R. W., Shannon, E. J. and Hastings, R. C. Host responses to the phenolic glycolipid-I antigen of M. leprae. Int. J. Lepr. 53(1985)710-711.
27. Waters, M. F. R., Ridley, D. S. and Ridley, M. J. Clinical problems in initiation and assessment of multidrug therapy. Lepr. Rev. 57 Suppl. 3(1986)92-100.
28. World Health Organization. WH O Study Group. Chemotherapy of Leprosy for Control Programmes. Geneva: World Health Organization, 1982. Tech. Rep. Scr. No. 657.
1. Ph.D., Research Officer.
2. M. D., Deputy Director.
3. M. Stat., Senior Rescarch Officer.
4. Ph.D., Deputy Director, Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India.
5. B.Sc., final year medical student on "elective period" from the University of Glasgow, Scotland.
Received for publication on 20 June 1988.
Acceptedfor publication in revised form on 1 November 1988.