- Volume 57 , Number 1
- Page: 33–7
A pilot study of three potential vaccines for leprosy in bombay
ABSTRACT
Three vaccines, BCG Glaxo alone (vaccine A), BCG Glaxo plus 107 killed Mycobacterium vaccae (vaccine B), and BCG Glaxo plus 107 killed M. leprae (vaccine C), were given to groups of selected children. The effects of these vaccines on subsequent quadruple skin testing 1-3 years after vaccination were compared. All three vaccines equally and significantly (p < 0.00001) increased positivity to tuberculin, but only vaccine B was found to significantly enhance development of skin-test positivity to leprosin A (p < 0.002). The data support the evidence previously obtained in rural Iran that the combination of BCG with killed M. vaccae is likely to be a better vaccine for leprosy than is BCG alone.RÉSUMÉ
On a administré à des groupes sélectionnés d'enfants trois vaccins, à savoir le BCG Glaxo seul (vaccin A), le BCG Glaxo accompagné de 107 Mycobacterium vaccae tués (vaccin B) et le BCG Glaxo plus 107 M. leprae tués (vaccin C). On a comparé les résultats d'une épreuve cutanée quadruple, pratiquée 1 à 3 ans après la vaccination, dans les trois groupes. Les trois vaccins ont, tous, eu comme effet d'accroître la réaction positive à la tuberculine, de manière statistiquement significative (p < 0.00001). Néanmois, seul le vaccin B renforcait signilicativement le développement d'une positivité cutanée à la léprosine A (p < 0.002). Ces données confirment les résultats obtenus précédemment dans les régions rurales de l'Iran, qui montraient qu'une combinaison de BCG et de M. vaccae tué paraît constituer un meilleur vaccin pour la lèpre que le BCG seul.RESUMEN
Se seleccionaron 3 grupos de niños a los cuales se les administró la vacuna BCG Glaxo sola (vacuna A), BCG Glaxo más 107 Mycobacterium vaccae muertos (vacuna B), o BCG Glaxo más 107 M. leprae muertos (vacuna C). Se compararon los efectos de estas tres vacunas sobre las respuestas intradérmicas de los niños a 4 antígenos de prueba, 1-3 años después de la vacunación. Las tres vacunas incrementaron significativamente (p < 0.00001) la reactividad a la tuberculina, pero sólo la vacuna B incrementó significativamente la reactividad a la leprosina A (p < 0.002). Los datos apoyan la evidencia obtenida previamente en Irán de que la combinación de BCG con M. vaccae muerto es probablemente una mejor vacuna contra la lepra que el BCG sólo.Trials of the efficacy of BCG vaccination against leprosy have produced variable results, but the study carried out in Uganda showed that under some conditions at least it could be very effective (2). The development of soluble preparations of leprosy bacilli for skin testing (4, 14) has meant that modified vaccines could be assessed without the expense of direct protection trials, on the assumption that the induction of positivity to these skin-test reagents indicates an improved level of immune protection. Thus, Convit and his colleagues have used their soluble leprosy reagent (4) to assess the effects of a combination of BCG plus killed Mycobacterium leprae as a vaccine (3). This vaccine is currently under trial as to its direct protective effect in South America and Malâwi.
The soluble reagent leprosin A, prepared in London (14) from leprosy bacilli, has been used to assess the effects of BCG in the Indian towns of Agra (10) and Ahmednagar (15), and to evaluate the combinations of BCG with M. vaccae or M. leprae in The Lebanon (1) and in Iran (5, 16). In the present study, we have used this reagent together with three other new tuberculins (9) to determine the effects of these combinations in children attending schools in the slums of Bombay, India. Bombay is an enormous city with 8-9 million inhabitants where both leprosy and tuberculosis are endemic. In the slums where the children live environmental mycobacteria abound, and even the piped drinking water may contain up to 108 mycobacteria per liter (6).
MATERIALS AND METHODS
Reagents used. The skin-test reagents used were four of the series of new tuberculins (9)-tuberculin, leprosin A, scrofulin, and vaccin-prepared from M. tuberculosis, M. leprae, M. scrofulaceum, and M. vaccae, respectively. They were given as 0.1 ml intradermal injections at least 10 cm apart two on each forearm. The doses injected were 0.2 µg of tuberculin and scrofulin, 1.0 µg of leprosin A, and 2.0 µg of vaccin, as used in previous studies (1, 5, 16). Diameters of induration were measured after 72 hr, and mean reaction sizes of 2 mm or more were considered positive. Our experience in other studies (5, 10, 14-16) led us to take this small size as positive. Proper use of the short bevel, 26-gauge, intradermal needle, which causes minimal trauma (rarely more than 1 x 1 mm) has not led to problems in the interpretation of small-sized reactions. The arbitrary decision that 4 x 5 mm is a negative response and 5x 5 mm a positive response has never seemed sensible to us, especially when biopsy data show that the same cells infiltrate very small reactions (and a proportion of zero reactions) as infiltrate large reactions (17).
The vaccines used were the same as those previously employed in studies in The Lebanon (1), and in Iran (5, 16). BCG Glaxo (freeze dried) was made up with the water provided by the manufacturer (vaccine A), or the same BCG was made up with a suspension of 108/ml of M. vaccae in saline (vaccine B), or with a suspension of 108/ml of M. leprae in saline (vaccine C). The organisms in these suspensions had been killed by exposure to 2.5 megarads from a60cobalt source. Vaccines were administered by an intradermal injection of 0.1 ml high over the left deltoid muscle.
Study plan. The children studied were aged 3-17 years and attended a number of schools in several different slum districts of the city. They were arbitrarily selected for vaccination on the basis of the lack of a scar from earlier BCG vaccination and a skintest reaction to tuberculin of less than 5-mm mean diameter of induration. Out of 1595 children tested, 395 were suitable for vaccination, and 292 were vaccinated-96 were given vaccine A, 99 were given vaccine B, and 97 were given vaccine C. Among the 1200 children who were not suitable for vaccination, 775 had BCG scars (523 or 67% of them were tuberculin positive) (11), and 425 had responses to tuberculin of 5 mm or more despite their lack of a BCG scar.
Follow-up skin testing with the four reagents was carried out 1 to 3 years after vaccination in as many children as possible, without the person doing the tests knowing which child had received which vaccine. Thus, the results were recorded "blind." The sizes of the vaccine scars were also measured at these times. Most of the skin testing and vaccinating for the study was carried out by a series of students in the course of their elective studies.
RESULTS
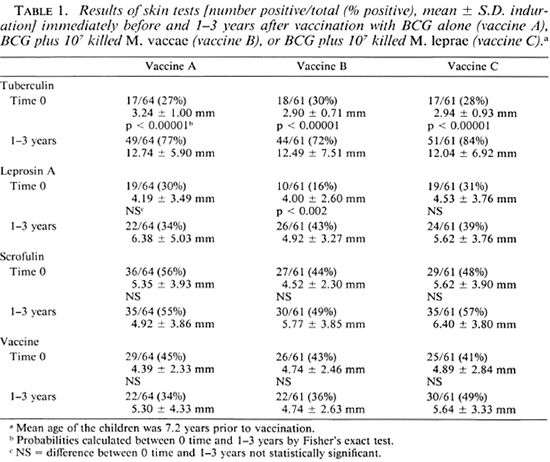
Effects of the three vaccines. The mean age at the time of vaccination of the children who were followed up was 7.2 ± 1.7 (mean ± S.D.) years (range 3-15 years), and the mean age of those among them who had responses to leprosin A at the time of vaccination was 7.0 ± 1.6 years (range 5-13 years). The skin-test results for these children before and from 1-3 years after vaccination with each of the three vaccines are shown in Table 1. It can be seen that all three vaccines significantly increased tuberculin positivity (p < 0.00001), and that only vaccine B increased leprosin A positivity (p < 0.002). There were no significant effects on responsiveness to scrofulin or vaccin.
The postvaccination scar sizes were the same for all three vaccines, and the mean scar size for all children was 6.1 ± 2.5 mm; two children had no visible scar, and the largest scar measured 17 mm. There were no significant differences between the different schools in the effects of the vaccines or between the scar sizes (data not shown).
DISCUSSION
All three vaccines very significantly increased tuberculin positivity (p < 0.00001) to a similar degree, showing that the additions did not adversely affect tuberculin conversion. The most important observation of the study is that the incorporation of M. vaccae with BCG (vaccine B) produced a significant enhancement of postvaccination leprosin-A positivity, which was not seen after BCG alone or after the combination of BCG with M. leprae. However, it has to be noted that there was no significant difference in the final percentage positivity to leprosin A after each of the three vaccines. Inclusion of an unvaccinated group in our study might have been desirable, but was not considered ethical.
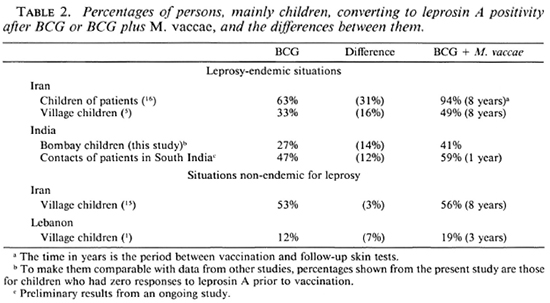
Leprosy is highly endemic for the slum areas where the schools were situated, and the source of sensitization to leprosin A is likely to be from casual contact with leprosy patients, rather than a direct effect of the vaccine. Thus, the data obtained in urban Bombay support the observations made in rural Iranian Azerbaijan (5, 16) that the combination of BCG Glaxo with M. vaccae may be a better vaccine for leprosy than is BCG alone (Table 2). Although the numbers vaccinated were small, it is interesting to note that the addition o M. leprae to BCG did not significantly improve recognition of leprosin A over the period of the study.
The mechanism of action of vaccines containing BCG is interesting in that postvaccination positivity directly caused by the vaccine wanes very considerably within 3 years unless boosted from the environment. This is clearly seen in the comparison between results obtained in The Lebanon (1), where contact with environmental mycobacteria appears to be minimal, and those obtained in Agra (10) and Ahmednagar (15), where environmental mycobacteria abound. BCG acts by priming the individual to recognize small environmental challenges and to develop delayed-type hypersensitivity, which may be directed to antigens of group i (common mycobacterial), group iv (7, 12, 13) (species specific), or even of group ii (8) (slowgrower associated antigens).
M. vaccae was specifically added to BCG to improve recognition of casually encountered organisms, not to induce positivity to leprosin A by some crossreactivity in species-specific (group iv) antigens between it and the leprosy bacillus. The present study is one of a series in which the effects of the addition of M. vaccae are being assessed in situations of differing leprosy endemicity. The results of studies completed or still in progress are shown in Table 2. In each of the studies in leprosy-endemic regions, skintest recognition of the leprosy bacillus has been successfully enhanced
Acknowledgments. We should like to thank the children, their parents, and the staff of the schools for their participation in this study. Without the assistance of members of the staff of the Bombay Leprosy Project we could not have carried out the work, and we are very grateful for their assistance.
We also wish to thank LEPRA for financial support for preparation of the reagents, and Glaxo Ltd. for the donation of BCG. Money was kindly provided by LEPRA which, together with bursaries from the University of Leicester and from the Medical Research Council, U.K., allowed the students to carry out their elective studies. We thank our supporters most sincerely.
REFERENCES
1. Bahr, G. M., Stanford, J. L., Rook, G. A. W., Rees, R. J. W., Abdulnoor, A. M. and Frayha, G. J. Two potential improvements to BCG and their effect on skin test reactivity in The Lebanon. Tubercle 67(1986)205-218.
2. Brown, J. A. K. , Stone, M. M. and Sutherland, I. B.C.G. vaccination of children against leprosy in Uganda; first results and second follow-up. Br. Med. J. 1(1966)7-14 and 1(1968)24-27.
3. Convit, J., Aranzazu, N., Ulrich, M., Zuniga, M., de Aragon, M. E., Alvarado, J. and Reyes, O. Investigations related to the development of a leprosy vaccine. Int. J. Lepr. 51(1983)531-539.
4. Convit, J., Pinardi, M. E., Avila, J. L. and A ranzazu, N. Specificity of the 48-hour reaction to Mitsuda antigen. Bull. WHO 52(1975)187-191.
5. Ghazi Saidi, K. , Stanford, J. L., Stanford, C. A., Dowlati, Y., Farshchi, Y., Rook, G. A. W. and Rees, R. J. W. Studies on the combination of BCG with Mycobacterium leprae in children living in villages with differing endemicity for leprosy. Int. J. Lepr. 57(1989)45-53.
6. Kazda, J., Ganapati, R., Revankar, C, Buchanan, T. M. and Irgens, L. M. Isolation of environment-derived Mycobacterium leprae from soil in Bombay. Lepr. Rev. 57 Suppl. 3(1986)201-203.
7. Lockwood, D. N. J., Mc Manus, I. C,Stanford, J. L., Thomas, A. and Aheyaounawardana, D. V. P. Three types of response to mycobacterial antigens. Eur. J. Respir. Dis. 71(1987)348-355.
8. McManus, I. C, Lockwood, D. N. J., Stanford, J. L., Shaaban, M. A., AbdulAti, M. and Bahr, G. M. Recognition of a category of responders to group ii, slow-grower associated antigens in Kuwaiti senior school children using a statistical model. Tubercle 69(1988)275-281.
9. Shield, M. J., Stanford, J. L., Paul, R. C. and Carswell, J. W. Multiple skin testing of tuberculosis patients with a range of new tuberculins, and a comparison with leprosy and Mycobacterium utcerans infection. J. Hyg.(London)78(1977)331-348.
10. Stanford, J. L., Cunningham, F., Pilkington, A., Sargeant, I., Series, H., Bhatti, N., Bennett, E. and Mehrotra, M. L. A prospective study of BCG given to young children in Agra, India-a region of high contact with environmental mycobacteria. Tubercle 68(1987)39-49.
11. Stanford, J. L., Ganapati, R., Revankar, C. R., Lockwood, D. N. J., Price, J., Ashton, P., Ashton, L. and Rees, R. J. W. Sensitisation by mycobacteria and the effects of BCG on children attending schools in the slums of Bombay. Tubercle 69(1988)293-298.
12. Stanford, J. L. and Grange, J. M. The meaning and structure of species as applied to mycobacteria. Tubercle 55(1974)143-152.
13. Stanford, J. L., Nye, P. M., Rook, G. A. W., Samuel, N. and Fairbank, A. A preliminary investigation of the responsiveness or otherwise of patients and staff of a leprosy hospital to groups of shared or species-specific antigens of mycobacteria. Lepr. Rev. 52(1981)321-327.
14. Stanford, J. L., Rook, G. A. W., Samuel, N., Madlener, F., Khamenei, A. A., Nemati, T., Modabber, F. and Rees, R. J. W. Preliminary immunological studies in search of correlates of protective immunity carried out on some Iranian leprosy patients and their families. Lepr. Rev. 51(1980)303-314.
15. Stanford, J. L., Sheikh, N., Bogle, G., Baker, C, Series, H. and Mayo, P. Protective effect of BCG in Ahmednagar, India. Tubercule 68(1987)169-176.
16. Stanford, J. L., Stanford, C. A., Ghazi Saidi, K., Dowlati, Y., Stefani, F., Farshchi, Y., Madlener, F. and Rees, R. J. W. Vaccination and skin test studies on the children of leprosy patients. Int. J. Lepr. 57(1989)38-44.
17. Swanson Beck, J., Morley, S. M. , Gibbs, R. C, Potts, M. I., Kardjito, T., Grange, J. M., Stanford, J. L. and Brown, R. A. The cellular responses of tuberculosis and leprosy patients and of healthy controls in skin tests to "New Tuberculin" and Leprosin A. Clin. Expcr. Immunol. 64(1986)484-94.
1. M.B.B.S, D.D.V., The Bombay Leprosy Project, Bombay, Maharashtra, India.
2. M.D., D.P.H., The Bombay Leprosy Project, Bombay, Maharashtra, India.
3. M.B.Ch.B., M.R.C.P., The School of Pathology, University College and Middlesex School of Medicine, London, U.K
4. M.B.B.S., M.R.C.P., The School of Pathology, University College and Middlesex School of Medicine, London, U.K
5. M.B.Ch.B., The School of Pathology, University College and Middlesex School of Medicine, London, U.K
6. M.B.Ch.B., The School of Pathology, University College and Middlesex School of Medicine, London, U.K
7. M.B.Ch.B., The School of Pathology, University College and Middlesex School of Medicine, London, U.K
8. M.B.Ch.B., The School of Pathology, University College and Middlesex School of Medicine, London, U.K
9. M.B.B.S., The School of Pathology, University College and Middlesex School of Medicine, London, U.K
10. M.D., The School of Pathology, University College and Middlesex School of Medicine, London, U.K;
11. M.B.B.S., F.R.C.P., F.R.C.Path., The Clinical Research Centre, Watford Road, Harrow, Middlesex, U.K.
Received for publication on 26 May 1988.
Accepted for publication in revised form on 3 November 1988.
Reprint requests to: Dr. J. L. Stanford, School of Pathology, University College and Middlesex School of Medicine, Riding House Street, London W1P 7LD, U.K.