- Volume 57 , Number 1
- Page: 38–41
Vaccination and skin test studies on the children of leprosy patients
Based on the assumption that skin-test reactivity to a soluble extract of leprosy bacilli (leprosin A) is an indicator of protective immunity from multibacillary leprosy in a population, we have sought means of inducing such reactivity in as many as possible of the children of leprosy patients. The children studied live with their parents in Baba Baghi Leprosy Sanatorium in eastern Azerbaijan (6). The study started in 1978 (15) and is continuing.
Since long before our study began, it had been the policy of the sanatorium to vaccinate infants, or children when they started school, with BCG Pasteur (produced in Teheran), and to give children between the ages of 5 and 10 years a weekly dose of 4 mg/kg of dapsone. We have assessed by quadruple skin testing the effects of BCG vaccination and of our own attempts to improve on it by the addition of suspensions of killed Mycobacterium vaccae.
MATERIALS AND METHODS
The children. With the very few exceptions of those children who were absent from the sanatorium at the time, all of the children were tested and entered into the study in 1978. In every case these children had at least one parent with multibacillary leprosy and, in many cases, both parents were affected. Among the patients, there are known to be some who did not take dapsone in the days of monotherapy and who still do not take multiple drug therapy (MDT). More than half of the children were born in the sanatorium, and others accompanied their parents from leprosy-endemic villages (15). Despite the frequency with which they must meet leprosy bacilli in the very close community in which they live, only one child born in the sanatorium had developed the disease (borderline tuberculoid) prior to 1978, and none have done so since. The ages of the children entering the study were from 1 to 21 years.
Reagents. The skin-test reagents used were leprosin A (1 jug protein/dose) produced from filtered ultrasonicate of leprosy bacilli extracted from armadillo tissues (4, 9, 15), and new tuberculins produced from various mycobacteria grown on Sauton's medium (10). A variety of new tuberculins were used in 1978, but only tuberculin (0.2 µg/dose), scrofulin (0.2 µg/dose), and vaccin (2 µg/dose) produced from M. tuberculosis, M. scrofulaceum, and M. vaccae, respectively, were used in 1986-1988 together with leprosin A. These reagents were injected intradermally into the volar aspects of the forearms, two tests per arm at least 10 cm apart. The results were read as diameters of induration 72 hr after injection. Responses of 2 mm or more in diameter were considered positive, although decisions to vaccinate were based on responses to leprosin A of less than 3 mm. In addition to the results for individual skin tests, we were able to determine the categories of responders (5, 6) on the bases of the 1986-1988 results. Category 1 individuals respond to common mycobacterial (group i) antigens (11, 13) in most cases; category 2 nonresponders have a skin-test suppressor mechanism in operation in most cases (5, 6, 20); category 3 individuals respond to the species-specific (group iv) antigens of species of which they have experience.
The vaccines used were BCG Glaxo (intradermal), 106 viable units per dose, made up in a saline suspension of killed M. vaccae, 107 per dose, called vaccine B as in The Lebanon (1), and a saline suspension of killed M. vaccae alone, 108 per dose, called vaccine D. These vaccines were administered as 0.1 ml intradermal injections high up over the left deltoid muscle. As previously described (1), M. vaccae strain R877R (NCTC 11659) was harvested from Sauton's medium, suspended in saline for use, distributed in 5-ml glass vials, and exposed to 2.5 megarads from a 60cobalt source. Following irradiation, batches were checked for sterility by culture.
Study plan. In 1978 all available children were skin tested with leprosin A and other new tuberculins, and searched for scars of previous BCG vaccination. Those who produced responses of less than 3x 3 mm to leprosin A were vaccinated with vaccine B if they did not already have a BCG scar, and with vaccine D if they did have a scar. In 1986, all the children who could be found of those we had vaccinated and a small group of those who had not needed vaccination were followed up by repeat skin testing. More children who had not needed vaccination were followed up in 1988. In 1987 and the spring of 1988, another scries of children were skin tested and, according to the results, vaccinated as described above. Fisher's exact test and Student's /test were used in the analysis of the results of the study.
RESULTS
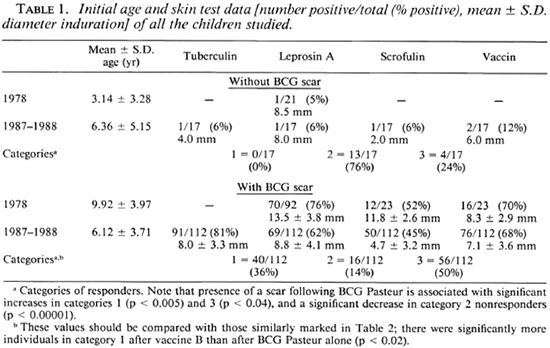
One hundred-thirteen children were tested in 1978; 20 of 21 without BCG scars were vaccinated with vaccine B (BCG + M. vaccae) and 22 of 92 with BCG scars were vaccinated with vaccine D (M. vaccae alone). An additional 129 children were tested in 1987-1988; all 17 of those without BCG scars were given vaccine B, and 44 of 112 with BCG scars received vaccine D. The initial ages and skin-test data for all these children are shown in Table 1. It can be seen that 70 (76%) of 92 children with BCG scars were positive reactors to leprosin A in 1978; whereas only 1 (4.8%) out of 21 children without BCG scars had a positive response at that time. Among the children first tested in 1986-1988, 70 (62.5%) of 112 children with BCG scars were leprosin A positive and 0 out of 17 without BCG scars had a positive response.
The skin-test data obtained in 1986-1988 from as many as possible of those vaccinated in 1978, and of a group of 29 children who did not require vaccination in 1978, are shown in Table 2. It can be seen that leprosin A positivity present in 1978 in these 29 had persisted, and that our additional vaccination procedures lead to a very high level of such positivity. After vaccine B, responsiveness to leprosin A was 15/16 (94%). Responsiveness to tuberculin, scrofulin, and vaccin was also high with 11 (69%) of the 16 category 1 responders (5, 6, 11) recognizing group i (11, 13) , common mycobacterial antigens. Only one child failed to react to all four skin-test reagents and can be considered a category 2 nonresponder (5, 11, 20) , Following vaccine D given to children who already had BCG scars but who were unresponsive to leprosin A, 14 (78%) out of 18 become leprosin A positive, 9 belonging to category 1 and, again, only one was a category 2 nonresponderto all four reagents. Of the 70 children with BCG scars who did not require vaccination in 1978, 29 were followed up in 1986-1988 and all were found still be to positive to leprosin A. The Figure shows the 1986-1988 leprosin A results in children with BCG scars according to their ages at the time they were tested. (It should be noted that it has been the habit at Baba Baghi to give (BCG) vaccination either soon after birth or soon after starting school at the age of 6 years.) Although there is no significant increase in percent positivity with age, there is a significant increase in the mean size of the response (p < 0.005, Student's t test). The same trends in response sizes are found with tuberculin and scrofulin (data not shown). The effects of our additional vaccination procedures are indicated in relation to what might have been expected had the children received BCG alone. It can be seen that after 8-10 years the overall result of giving vaccine B to children without previous BCG scars is the same as that of BCG Pasteur supplemented by giving vaccine D to those who where leprosin A negative.
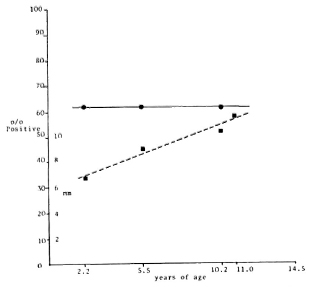
The figure. EiTects cif-age on percentage positivity( ) and response sizes (
) and response sizes ( ) to leprosin A in children who had received BCG, and the effects of the two new vaccines, measured in 1986-1988. (-) = no cant change in percentage positivity after BCG Pasteur(Teheran) with age. Percentage positivity values forboth vaccine B and vaccine D given after BCG Pasteur are shown as the separate solid circles. In both cases these are significantly higher than those obtained with BCG Pasteur alone (p < 0.005). Value shown for vaccine D is the combined total of those positive in 1978 after BCG Pasteur and those positive in 1986 after vaccine D. (- - - ) = increasing size of positive responses after BCG Pasteur with age of those first testedin 1986-1988. Older children (mean age 10.2 years)produced significantly larger responses (p < 0.005, Student's t test) than did younger children (mean age 2.2 years). The highest solid square shows mean size of response to leprosin A after vaccine B.
) to leprosin A in children who had received BCG, and the effects of the two new vaccines, measured in 1986-1988. (-) = no cant change in percentage positivity after BCG Pasteur(Teheran) with age. Percentage positivity values forboth vaccine B and vaccine D given after BCG Pasteur are shown as the separate solid circles. In both cases these are significantly higher than those obtained with BCG Pasteur alone (p < 0.005). Value shown for vaccine D is the combined total of those positive in 1978 after BCG Pasteur and those positive in 1986 after vaccine D. (- - - ) = increasing size of positive responses after BCG Pasteur with age of those first testedin 1986-1988. Older children (mean age 10.2 years)produced significantly larger responses (p < 0.005, Student's t test) than did younger children (mean age 2.2 years). The highest solid square shows mean size of response to leprosin A after vaccine B.
Like BCG, vaccine B forms a small ulcer 2 to 3 weeks after its injection and leaves a small permanent scar at the site. The mean ± S.D. size of the scar in the children we vaccinated was 4.3 ± 1.4 mm (range 2 to 6 mm). It should be noted that this is no different from the size of scar following this vaccine in children in The Lebanon who were not known to be in contact with any mycobacterial disease (1). Vaccine D occasionally ulcerates and leaves a small scar, rarely larger than 2 mm when measured a year or more later.
DISCUSSION
Plenty of evidence has been published that skin-test responses to soluble antigens of leprosy bacilli rarely accompany multibacillary leprosy ( 3 - 9 ) , and there is no evidence that healthy individuals responding to such reagents ever develop this type of disease. In view of this, it seems a reasonable assumption that a long-lasting high level of responsiveness to soluble antigens of M. leprae in a BCG-vaccinated population indicates protective immunity to multibacillary disease. Thus, the highly significant differences (p < 0.0005, Fisher's exact test) in leprosin A positivity between individuals with and without scars of vaccination with BCG Pasteur (Teheran) may be evidence supporting the value of this vaccine in the prevention of multibacillary disease in Baba Baghi. BCG vaccination is also accompanied by significant increases in category 1 (p < 0.005) and category 3 (p < 0.04) responders, at the expense of a marked reduction in category 2 (p < 0.00001) nonresponders (Table 1). The question to be answered is can this be further improved.
There are two obvious approaches to this problem: a) modification of the vaccine, and b) selection of the age at which it should be administered. Earlier work has shown that the optimal age for BCG vaccination depends on the child's environment (12, 16), probably the mycobacteria in it (14 ), and that the vaccine is only likely to be effective if given before infection becomes established(17, 18) . In view of the special conditions of a leprosy sanatorium, early vaccination would seem desirable.
The conceptual framework on which the combination of BCG plus killed M. vaccae is based is that M. vaccae provides additional common mycobacterial (group i) antigen (13) and also certain immunosuppressive determinants (7) which may favor the non-necrotizing type of cellular response to mycobacteria (8) and perhaps militate against tissue-damaging reactions. There is plenty of evidence that recognition of group i antigens is essential to protective immunity from clinical disease (14), and obviously the induction of necrotizing responses to mycobacteria in an individual with a large bacillary load might prove catastrophic. Leprosin A positivity may indicate recognition of group i antigens in category 1 responders (persons producing positive responses to all tuberculins (5, 11) as well as recognition of species-specific (group iv) antigens of the leprosy bacillus. These latter are not in the vaccines, and therefore the stimulus to recognize them must come from the environment, explaining why postvaccination positivity varies with the environment (2). The
1. M.D., School of Pathology, University College and MiddlesexSchool of Medicine, London W1P 7LD, U.K.;
2. S.R.N., School of Pathology, University College and MiddlesexSchool of Medicine, London W1P 7LD, U.K.;
3. D.V.M., M.P.H., School of Public Health, Teheran University, Teheran, Iran.;
4. M.D., Ph.D., Iran Leprosy Organization, Teheran, Iran.,
5. Sr. F. Weiss, National Institute for Medical Research, Mill Hill, London, U.K.
6. Y. Farshchi, National Institute for Medical Research, Mill Hill, London, U.K.
7. Baba Baghi Leprosy Sanatorium, Azerbaijan, Iran., National Institute for Medical Research, Mill Hill, London, U.K.
8. M.B.B.S., F.R.C.P., F.R.C.Path., National Institute for Medical Research, Mill Hill, London, U.K.
Received for publication on 21 March 1988; accepted for publication in revised form on 3 November 1988.