- Volume 57 , Number 1
- Page: 45–53
Vaccination and skin test studies on children living in villages with differing endemicity for leprosy and tuberculosis
ABSTRACT
The purpose of this study carried out in Iranian Azerbaijan was to determine the pattern of skin-test positivity to mycobacterial antigens in children living in the valley, and to assess the effect on this of a scries of vaccines against mycobacterial disease. Set up in 1978, 1707 tuberculin-negative children without scars of previous BCG vaccination were vaccinated with BCG Glaxo alone (vaccine A) or with the addition of a suspension of killed Mycobacterium vaccae (vaccine B). One hundred children were vaccinated with BCG Glaxo plus a suspension of M. leprae (vaccine C). Eight to 10 years later about half of the children were found for follow up. At this time further children were skin tested, and the results obtained were related to whether or not they had scars of vaccination with BCG Pasteur (Teheran) given by the local health authorities.Between setting up the study and the first follow up, cases of leprosy or tuberculosis had occurred in some of the villages, although not among those we had vaccinated. Differences between the efects of the vaccines were only found in villages with cases of leprosy. In these villages positivity to leprosin A was significantly greater after vaccine B (49%) than after vaccine A (36%; p < 0.04). The results for scrofulin and vaccin were the same after both vaccines, and significantly lower than in the villages without cases of leprosy. The general reduction in skin-test positivity in the villages with leprosy cases was mainly due to a loss of category 1 responders to group i, common mycobacterial, antigens.
It was concluded that where casual contact with cases of leprosy occurs the combination of BCG with killed M. vaccae is likely to be a better vaccine for leprosy than is BCG alone. Although few children received the combination with M. leprae, the results obtained were not particularly promising.
RÉSUMÉ
Le but de cette étude, menée dans l'Azerbeidjan iranien, a été de déterminer le profil de la réaction positive à l'épreuve cutanée à des antigènes mycobactériens chez des enfants vivant dans la vallée, et d'évaluer les ctléts sur cette réponse positive d'une série de vaccins contre la maladie mycobactérienne. En 1978, 1707 enfants négatifs à la tuberculine, et sans cicatrice d'une vaccination antérieure au BCG, ont été vaccinés par le BCG Glaxo uniquement (vaccin A), ou par le BCG Glaxo additionné d'une suspension de Mycobacterium vaccae tués (vaccin B). De plus, 100 enfants ont été vaccinés par le BCG Glaxo accompagné d'une suspension de M. leprae (vaccin C). Huit à dix ans plus tard, on a réussi à retrouver environ la moitié de ces enfants. A ce moment, on a pratiqué des épreuves cutanées chez d'autres enfants. Les résultats observés ont été mis en relation avec l'existence ou non de cicatrices de vaccination par le BCG Pasteur (Téhéran) qui avait été administré par les autorités locales de la santé.Entre le début de l'étude et le premier examen de suivi, des cas de lèpre ou de tuberculose sont survenus dans certains des villages, mais non parmi les enfants qui avaient été vaccinés. Des différences entre les effets de ces vaccins n'ont été constatées que dans les villages où étaient apparus des cas de lèpre. Dans ces villages, une réaction positive à la léprosine A était signilicati vement plus fréquente après le vaccin B (49%) qu'après le vaccin A (36%) (p < 0,04). Les résultats pour la scrofuline et le vaccin étaient similaires pour les deux vaccins, et les réactions étaient signilicativement plus faibles que dans les villages où n'étaient apparus aucun cas de lèpre. La réduction globale d'une réaction cutanée positive dans les villages avec des cas de lèpre était due principalement à une perte de répondeurs de catégoric 1, c'cst-à-dire de personnes qui répondent aux antigènes mycobactériens communs du groupe i.
On en conclut que dans les situations où les contacts occasionnels avec des cas de lèpre peuvent se produire, la combinaison de BCG avec M. vaccae tué est vraisemblablement un meilleur vaccin contre la lèpre que ne l'est le BCG seul. Encore qu'un petit nombre seulement d'enfants ait reçu la combinaison avec M. leprae, les résultats ne sont pas particulièrement prometteurs.
RESUMEN
El propósito de este estudio realizado en Azerbaijan, Irán, fue el determinar el patrón de reactividad en piel contra antígenos micobacterianos en los niños del lugar y el de medir el efecto de vacunas anti-micobacterianas sobre el patrón de reactividad. En 1978, 1707 niños tuberculino negativos, sin la cicatriz de la vacunación previa con BCG, se vacunaron con BCG Glaxo sola (vacuna A) o adicionada de una suspensión de M. vaccae muerto (vacuna B). Adicionalmente, cien niños fueron vacunados con BCG Glaxo más una suspensión de M. leprae (vacuna C). Ocho a diez años después, la mitad de los niños fueron localizados y evaluados. En este momento se incluyeron otros niños a quienes se les hicieron pruebas en piel, y los resultados obtenidos se relacionaron con la presencia o ausencia de cicatrices por vacunación con BCG Pasteur (Teherán) aplicada por las autoridades locales de salud.Entre el comienzo del estudio y la primera evaluación, aparecieron casos de lepra o tuberculosis en alguna de las poblaciones, pero no en aquellas que nosotros habíamos vacunado. Las diferencias entre los efectos de las vacunas sólo se encontraron en las poblaciones con casos de lepra. En estas poblaciones, la positividad a la leprosina A fue significativamente mayor después de la vacuna B (49%) que después de la vacuna A (36%; p < 0.04). Los resultados para escrofulina y vaccina fueron iguales antes de ambas vacunas, y significativamente más bajos que en las poblaciones sin casos de lepra. La reducción general en las positividad dérmica en las poblaciones con casos de lepra se debió principalmente a la pérdida de respondedores de la categoría 1 a los antígenos i, comunes entre las micobacterias.
Se concluyó que para los contactos casuales con pacientes con lepra, la combinación BCG con M. vaccae muerto es mejor vacuna contra la lepra que el BCG sólo. Aunque algunos niños recibieron la combinación de BCG con M. leprae, los resultados obtenidos no fueron particularmente prometedores.
The purpose of this study was to investigate the potential value of several vaccines that might provide protective immunity from leprosy and tuberculosis. These consisted of two preparations of BCG vaccine and two additives that might enhance the efficacy of the vaccine. Although the best, and some people would say the only, measure of protective immunity following a vaccine is the number of cases of disease that its use prevents, this is far too expensive for preliminary assessments, and would require far more staff and facilities than were available to us. Indirect correlates of protection are required that can be applied in the field to indicate the likely benefit. For operational reasons, the only indirect tests that could reasonably be used to select individuals for vaccination and to assess its subsequent effects are skin tests or serological tests. For our studies, we have used four skin tests including tuberculin and a preparation of the soluble antigens of Mycobacterium leprae known as leprosin A (13).
Studies on children of leprosy patients living with their parents in the Baba Baghi Leprosy Sanatorium near Tabriz in Iranian Azerbaijan have shown that long-lasting (8-9 years) skin-test positivity to leprosin A develops in about 60% of those given BCG vaccine, and that this can be enhanced to above 90% by the use of a suspension of killed M. vaccae (16). Much of this increased positivity is due to responses to group i, common mycobacterial antigens (11).
The present study has been carried out in children with varying contacts with leprosy living in villages in the Ahar Valley in eastern Azerbaijan, where there is a prevalence of leprosy of about 2.5 per thousand (6). This is the region from which many of the patients in Baba Baghi Leprosy Sanatorium originate. Between setting up the study in 1978 and follow-up in 1986-1988, the Ahar health office recorded new cases of leprosy in seven villages studied and new cases of tuberculosis in four villages. Thus, to some extent, we can assess the vaccines under different conditions of contact with disease.
MATERIALS AND METHODS
Population studied. Healthy children aged 2-16 years living in 59 villages in the Ahar Valley about 150 km northeast of Tabriz 1500 m above sea level were studied. The valley is bisected lengthwise by the Tabriz-Ahar-Meshkinshah road running close to the little Ahar River. As well as the town of Ahar itself, there are more than 900 villages in the valley, only some of which contain cases of leprosy. Although tuberculosis is not common in the villages, sporadic cases occur throughout the valley.
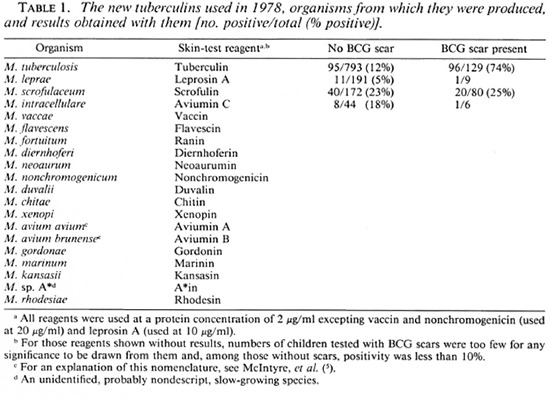
Reagents. The skin-test reagents used were new tuberculins prepared from the species shown in Table 1 at the concentrations indicated (3, 10). Each reagent was administered by an intradermal injection of 0.1 ml into the forearm. Results were read by measuring diameters of induration 72 hr after injection. For the analysis of results, mean diameters of induration of 2 mm or more were taken as positive.
The vaccines used were BCG Glaxo (intradermal) made up with water for injection (vaccine A), and the same concentration of BCG made up with a suspension of irradiation-killed M. vaccae strain R877R (NCTC 11659) in saline (vaccine B), or with a suspension of M. leprae harvested from experimentally infected armadillos (vaccine C) (1). The injected dose of 0.1 ml contained 106 viable units of BCG alone, or together with 107 killed M. vaccae or 107 killed M. leprae. Additionally, a suspension containing 108 killed M. vaccae alone in 0.1 ml of saline (vaccine D) was used in some children in 1986-1988 (16). Vaccines were always injected high up over the left deltoid muscle.
The fourth vaccine in the study was BCG Pasteur, prepared in Teheran and given by the local health workers to a variable proportion of infants. This vaccine was usually given over the right deltoid muscle. We did not try to influence this vaccination policy by our own studies, but we were able to assess its effects to some extent.
Study plan. In 1978, children in 27 of the villages were skin tested with two reagents, one test on each arm, the left one always being tuberculin. The other tests were with the various other reagents. The tests were read in 922 children. In view of the low level of tuberculin positivity found, vaccination was carried out without preliminary skin testing in a further 32 villages.
All children were carefully examined on both shoulders for BCG scars and the few possessing them, together with those few among those skin tested who had indurated responses to tuberculin greater than 5 mm in diameter, were not vaccinated. The remaining children in 57 of the villages received either vaccine A or vaccine B in a random manner. In two villages (Choopenlar and Pestebagloo) vaccines A, B, and C were used: 834 children received vaccine A, 873 received vaccine B, and 100 received vaccine C. Local lesions in a small number of recipients of each vaccine were examined a month later.
In 1986-1988, the follow-up studies were carried out "blind," that is, without knowledge of which vaccine each child had received. As many as possible of the children vaccinated in 1978 were examined for vaccine scars, and the sizes of these scars were measured. The children were also tested with tuberculin, leprosin A, scrofulin and vaccin, two tests on each forearm at least 10 cm apart. Because of the long time between setting up the study and its first follow up, only the scrofulin was of exactly the same batch as that used in 1978.
In the villages where new cases of leprosy or tuberculosis had occurred, and in two villages (one of them Pestebagloo) without new cases of mycobacterial disease, groups of children aged 2 to 16 years who were not included in the 1978 study were examined for BCG scars and tested with these same four reagents to assess the effects of BCG Pasteur (Teheran) given by the local health authorities. Any children tested who had responses to tuberculin of less than 5 mm and did not have a scar of previous BCG were given BCG Pasteur in 1986 and vaccine B in 1987-1988. Children with BCG scars or those who had tuberculin reactions of 5 mm or more without a BCG scar, but who had reactions to leprosin A of less than 3 mm were given vaccine D as used in similar children living in Baba Baghi (16).
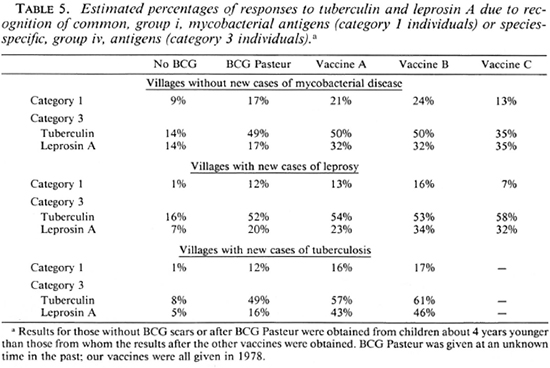
Analysis of results. Skin-test results were analyzed by percent positivity and mean positive reaction size for each of the followup reagents, and by responder categorization (4, 7). Category 1 individuals who respond to all four skin tests, may do so by recognition of group i, common mycobacterial, antigens (true category 1 responders), or by chance recognition of the group iv, species-specific antigens of all four. The number of these chance responders can be calculated, and the observed proportion of category 1 responders can be corrected by subtraction. This correction has been used to obtain the values shown in Table 5. Vaccine scar sizes were analyzed according to the vaccine given, and the village cluster from which the children came. The significance of the results was determined using Fisher's exact test and Student's t test, as appropriate.
RESULTS
The initial skin-test results obtained in 1978 are shown in Table 1. It can be seen that only 14% of children had scars of BCG Pasteur (Teheran), and that only 12% of those without scars were positive to tuberculin. The great majority of these tuberculin reactions were less than 5 mm in diameter. Among those without BCG scars, 6% were positive to leprosin A, 23% were positive to scrofulin, 18% were positive to aviumin C, and there was less than 10% positivity to each of the other reagents. The results obtained with tuberculin and leprosin A do not differ significantly from those obtained in 1986-1988 (Table 4).
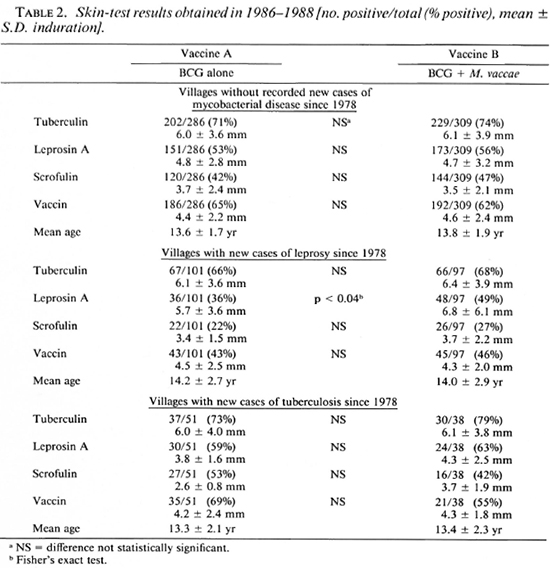
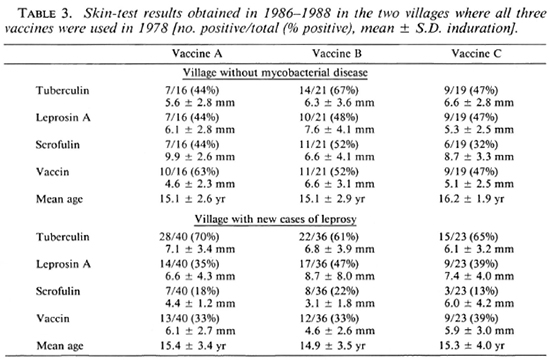
In 1986-1988, 54 of the villages were visited for follow-up skin testing and 438 children given vaccine A, 444 given vaccine B, and 42 given vaccine C were found, almost exactly half of the total number vaccinated in 1978. The sexes were equally distributed among those followed up. Table 2 shows the results obtained for vaccines A and B according to whether the children lived in villages with new cases of leprosy or tuberculosis occurring since 1978 or in those thought to be free of these diseases. It happened that vaccine C had been given in one village with new cases of leprosy (Choopenlar) and one without mycobacterial disease (Pestebagloo). The results for these two villages alone are shown in Table 3.
In the villages without disease or with tuberculosis, there were no statistically significant differences in skin-test positivity between those given the different vaccines. However, there was a tendency to higher positivity to all reagents after both vaccines A and B in the villages with tuberculosis. In the villages with new cases of leprosy, there was a tendency toward lower levels of positivity than in the other villages. These differences were statistically significant for all reagents other than tuberculin after vaccine A (p < 0.01), and for scrofulin and vaccin after vaccine B (p < 0.04). In the leprosy villages, vaccine B recipients were significantly more likely to respond to leprosin A than were vaccine A recipients (p < 0.04); this was only a nonsignificant trend in the other villages. There was also a tendency for skin-test responses to be slightly larger after vaccine B than after vaccine A, although this was not significant by Student's t.
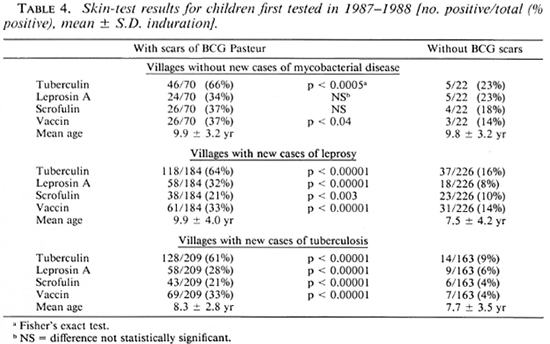
The skin-test results obtained in 1986-1988 from children who were not included in the 1978 study are shown in Table 4. The proportion with scars of BCG Pasteur (Teheran) was 463/874 (53%) in 1986-1-988 as compared with 129/922 (14%) found in 1978, indicating the increased use of the vaccine in the valley. There were differences in the results from children without BCG scars in the different village groups. Positivity to all four reagents was greatest in villages without disease, but the children tested were few in number and, on the average, more than 2 years older than those in the other villages; this may explain the differences. Children without BCG scars in the villages with cases of leprosy were significantly more responsive to tuberculin (p < 0.02), scrofulin (p < 0.02), and vaccin (p < 0.002) than were children in villages with cases of tuberculosis, despite their ages being the same. The results for children with scars of BCG Pasteur were very similar in all three village clusters except that positivity to scrofulin was greater in the villages without disease than in the other villages (p < 0.003).
Vaccine scar sizes. Vaccine scar sizes varied from 2-13 mm in mean diameter, and there were no significant differences in the sizes of scars after vaccines A or B, 4.43 ± 1.70 mm and 4.48 ± 2.10 mm, respectively. There was no evidence of local complications in anyone, but there was some variation in scar sizes between villages. For the two villages where vaccines A, B, and C were used the scar sizes were 5.87 ± 1.59 mm, 5.40 ± 2.69 mm and 4.63 ± 2.08 mm, respectively, for Pestebagloo (no mycobacterial disease) and 6.24 ± 2.51 mm, 6.75 ± 2.60 mm and 5.72 ± 2.42 mm, respectively, for Choopenlar (new cases of leprosy). The trend indicated by the larger scars in Choopenlar became statistically significant for vaccines A and B when the data from all the villages without mycobacterial disease were compared with that from all seven villages with new cases of leprosy. For vaccine A these were 4.28 ± 1.66 mm and 4.85 ± 2.33 mm, respectively; for vaccine B they were 4.29 ± 2.00 mm and 4.99 ± 2.36 mm, respectively (p < 0.01 by Student's t test in both cases). The presence of tuberculosis in a village did not significantly influence post-BCG scar sizes.
DISCUSSION
Although our study would have been better if we could have followed up a larger number of the children we vaccinated, we were very pleased to have found half of them after 8 to 10 years. A simple census carried out in several of the villages showed that about 60% of the children were still living in the villages and that the few who missed follow up were either away herding sheep, attending markets, or carrying out their military service. A few of the remaining 40% had died, many girls had left their village to get married, and many boys had left to obtain work in Tabriz or Teheran.
The skin-test data obtained in 1978 (Table 1) show that sensitization by environmental mycobacteria prior to BCG vaccination is generally low in the valley, with less than 10% positivity to 16 of the 18 reagents produced from these organisms (8). The only exceptions were the reactions to scrofulin and aviumin C. Positivity to leprosin A and tuberculin were also low. These results are very similar to those reported from The Lebanon (2), and very different from those reported from India (11, 14) .
Our results show an unexpected difference between children living in villages with new cases of leprosy and those living in villages with tuberculosis cases or without mycobacterial disease. In the villages with cases of leprosy, the children were less responsive to skin tests with reagents other than tuberculin after vaccine A than were the children in other villages. The results also show that in the villages with new cases of leprosy responsiveness to leprosin A is significantly greater after vaccine B than after vaccine A (p < 0.04) or after BCG Pasteur (p < 0.002). Although the numbers were too few to reach statistical significance, the trend was seen in Choopenlar (Table 3) after vaccine B but not after vaccine C incorporating M. leprae.
There was increased positivity to all skintest reagents after vaccine A in comparison with that following BCG Pasteur in the villages without new cases of disease or with new cases of tuberculosis. This could be due to differences in the ages of the children when the vaccines were given (generally younger for BCG Pasteur), or to the 5-year difference in ages at the time of skin testing. However, in the villages with new cases of leprosy the results for leprosin A and scrofulin after vaccine A are not different (p < 0.8 and p = 0.65, respectively) from those after BCG Pasteur despite the difference in ages. Vaccine B, on the other hand, resulted in significantly greater positivity to leprosin A than did BCG Pasteur (p < 0.002) in these villages.
These observations support the view that the children living in villages with new cases of leprosy are different from children living in the other villages (9) in their reactions to skin tests after vaccination. The increased size of post-vaccination scars in the villages with leprosy cases also supports this suggestion. It is difficult to be sure whether the observed differences are due to contact with leprosy bacilli or, perhaps, to the underlying reasons for the endemicity of leprosy in these particular villages. If they are due to contact with leprosy patients, we should have expected to find similar changes in the children studied in Baba Baghi Leprosy Sanatorium (16), which we did not. Since the villages are only separated from each other by one or two kilometers, and marriages between people living in different villages are quite common, it is difficult to believe that the differences are genetic in origin. A rational explanation is that the observed differences are due to environmental factors, probably the differing distribution of environmental mycobacteria (15).
To elucidate further the effects of the different vaccines, Table 5 shows the distributions of skin-test responses to common mycobacterial, group i, antigens (category 1 responders) and to species-specific, group iv, antigens (category 3 responders) in each of the village clusters. Category 1 is always found to be largest among children receiving vaccine B, and lowest in the non-vaccinated. Although the numbers followed up after vaccine C were few, it may be noticed that there were fewer category 1 responders after this vaccine than in any of the other vaccine groups, including those who received BCG Pasteur, despite the age difference of the children. However, recognition of species-specific antigens of M. leprae by category 3 children is the same after vaccines A, B, or C in the villages without disease, and as good as vaccine B in the villages with new cases of leprosy. It is interesting to note that recognition of leprosin A by the species-specific antigens it contains is greater after both vaccines A and B in the villages with new cases of tuberculosis than in the villages with new cases of leprosy.
Several tentative conclusions can be drawn from this study. BCG vaccination significantly increases tuberculin positivity. This increase persists for at least 8-10 years, and it is not significantly influenced by either of the additives tested. There are differences in the ways that children respond to skin tests after vaccination with BCG, according to the villages in which they live. These differences appear to be due to environmental factors. Both Glaxo BCG and Pasteur BCG produce a considerable increase in leprosin A skin-test positivity, and this is significantly increased by the incorporation of killed M. vaccae with BCG Glaxo if the children live in villages where there have been new cases of leprosy diagnosed during the last few years. This enhancement associated with the addition of M. vaccae is due to increased responsiveness to both common mycobacterial and species-specific antigens. Preliminary data obtained from the use of BCG Glaxo incorporating a suspension of killed leprosy bacilli suggest that this combination may enhance recognition of species-specific antigens of M. leprae but decrease recognition of mycobacteria by their common antigens. If the latter is important, M. vaccae may be a better additive to BCG in a vaccine for leprosy than is the leprosy bacillus itself.
Acknowledgments. We should like to thank the children and their parents in the villages for agreeing to participate in our study. Our thanks are also due to Mr. H. Ali Yasin and Mr. M. Saphari of the health olfice in Ahar, the village health workers, and to the skillful drivers of our vehicles. Our study could not have been carried out without the kind interest of Dr. A. Velayati, Minister of Foreign Affairs of the Islamic Republic of Iran. We are very grateful to him.
Financial support, for which we are very thankful, was provided by the Iran Leprosy Organization and the British Leprosy Relief Association (LEPRA). Glaxo Operations, U.K.Ltd. was kind enough to provide the BCG vaccine free of charge, and we are grateful to them.
REFERENCES
1. Bahr, G. M. , Stanford, J. L., Rook, G. A. W., Rees, R. J. W., Abdulnoor, A. M. and Frayha, G. J. Two potential improvements to BCG and their effect on skin test reactivity in The Lebanon. Tubercle 67(1986)205-218.
2. Bahr, G. M. , Stanford, J. L., Rook, G. A. W., Rees, R. J. W., Frayha, G. J. and Abdulnoor, A. M. Skin sensitisation to mycobacteria amongst school children, prior to a study of BCG vaccination in North Lebanon. Tubercle67(1986)197-203.
3. Draper, P. and Rees, R. J. W. Proposed system for preparing purified suspensions of M. leprae from tissues of infected armadillos. In: Report of the Second IMMLEP Task Force Meeting, December 1975, Protocol No. 2/75. Lepr. Rev. 47(1976)320-323.
4. Lockwood, D. N. J., Mc Manus, I. C, Stanford, J. L., Thomas, A. and Abeyagunawardana, D. V. P. Three types of response to mycobacterial antigens. Eur. J. Respir. Dis. 71(1987)348-355.
5. McIntyre, G., Belsey, E. and Stanford, J. L. Antigenic relationships reflecting taxonomic differences between Mycobacterium avium and M. inlracellulare elucidated in man by skin tests with three new tuberculins. Eur. J. Respir. Dis. 69( 1986)142-149.
6. Nasseri, K. and Ko, Y. H. Epidemiology of leprosy in Iran. Int. J. Lepr. 45(1977)355-359.
7. Nye, P. M. , Stanford, J . L., Rook, G. A. W., Lawton, P., MacGregor, M., Reily, C, Humber, D., Orege, P., Revankar, C. R., Terencio d e las Aguas, J. and Torres, P. Suppressor determinants of mycobacteria and their potential relevance to leprosy. Lepr. Rev. 57(1986)147-157.
8. Shield, M. J. The importance of immunologically effective contact with environmental mycobacteria. In: The Biology of the Mycobacteria, Volume 2. Ratledgc, C. and Stanford, J. L., cds. London: Academic Press, 1983, pp. 343-415.
9. Shield, M. J. and Stanford, J. L. The epidemiological evaluation in Burma of skin test reagent LRA6; a cell-free extract from armadillo-derived Mycobacterium leprae. Part 2: Close contacts and non-contacts of bacilliferous leprosy patients. Int. J. Lepr. 50(1982)446-454.
10. Shield, M. J., Stanford, J., L., Paul, R. C. and Carswell, J. W. Multiple skin testing of tuberculosis patients with a range of new tuberculins, and a comparison with leprosy and Mycobacterium ulcerans infection. J. Hyg.(London)78(1977)331-348.
11. Stanford, J. L., Cunningham, F., Pilkington, A.,Sargeant, I.,Series, H., Bhatti, N., Bennett, E. and Mehrotra, M. L. A prospective study of BCG given to young children in Agra, India-a region of high contact with environmental mycobacteria. Tubercle 68(1987)39-49.
12. Stanford, J. L. and Grange, J. M. The meaning and structure of species as applied to mycobacteria. Tubercle 55(1974)143-152.
13. Stanford, J. L., Rook, G. A. W., Samuel, N. , Madlener, F., Khamenei, A. A., Nemati, T., Modabber, F. and Rees, R. J. W. Preliminary immunological studies in search of correlates of protective immunity carried out on some Iranian leprosy patients and their families. Lepr. Rev. 51(1980)303-314.
14. Stanford, J. L., Sheikh, N. , Bogle, G., Baker, C, Series, H. and Mayo, P. Protective effect of BCG in Ahmcdnagar, India. Tubercule 68(1987)169-176.
15. Stanford, J. L., Shield, M. J. and Rook, G. A. W. How environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercule 62(1981)55-61.
16. Stanford, J. L., Stanford, C. A., Ghazi Saidi, K., Dowlati, Y., Stefani, F., Farshchi, Y., Madlener, F. and Rees, R. J. W. Vaccination and skin test studies on the children of leprosy patients. Int. J. Lepr. 57(1989)38-44.
1. D.V.M., M.P.H., School of Public Health, University of Teheran, Teheran, Iran;
2. M.D., School of Pathology, University College and Middlesex School of Medicine, London W1P 7LD, U.K.;
3. S.R.N., School of Pathology, University College and Middlesex School of Medicine, London W1P 7LD, U.K.;
4. M.D., School of Pathology, University College and Middlesex School of Medicine, London W1P 7LD, U.K.;
5. M.D., Ph.D., The Leprosy Association of Iran, Teheran, Iran;
6. Baba Baghi Leprosy Sanatorium, Tabriz, Iran;
7. M.B.B.S., F.R.C.P., F.R.C.Path., Clinical Research Centre, Harrow, Middlesex, U.K.
Received for publication on 21 March 1988.
Accepted for publication in revised form on 3 November 1988.
Reprint requests to: Dr. K. Ghazi Saidi, School of Pathology, University College and Middlesex School of Medicine, London W1P 7LD, U.K.