- Volume 57 , Number 1
- Page: 54–64
New data on the ultrastructure of the membrane of Mycobacterium leprae
ABSTRACT
In previous reports on the ultrastructure of Mycobacterium leprae, we described the occurrence of symmetric membranes innormal-looking bacilli f rom fresh or frozen samples primarily fixed with aldehydes. In those reports we admitted that such a symmetric profile, which is not found in the other normal mycobacteria, would not represent the structure of the normal membrane of the leprosy bacillus. We, therefore,reanalyzed the ultrastructure of the membrane of M. leprae. In the present work themicromorphology of the M. leprae membrane was studied by transmission electron-microscopy after the fixation of fresh samples by OsO4 plus calcium followed by glutaraldehyde plus formaldehyde and calcium followed by uranyl acetate. The study of samples f rom two patients with lepro-matous (LL) leprosy, three armadillos with natural leprosy, and one nude mouse with experimental leprosy showed that normal-looking bacilli present in lead-stained sections had asymmetric membranes with a thickness of 6.49 ± 0.36 nm. These membranes showed periodic acid-Schiff (PAS)-positive components exclusively located in the outer half of the bilayer. We demonstrated that the symmetric profile of the M.leprae membrane described in our previous reports corresponds, as admitted in those reports, to an abnormal membrane structure. Such an abnormality was now found to result f rom the use of primary fixation with aldehydes or of samples stored frozen before fixation. These results indicate that, although ultrastructurally similar to that ofthe other mycobacteria, the membrane of M. leprae has a peculiar sensitivity to fixation by aldehydes. Such a characteristic, which was not found in M. lepraemurium, M. aurum, M. avium, and M. tuberoldosis H37Ra, must reflect a unique membrane molecular structure, which is presently un-known.RÉSUMÉ
Dans les rapports précédents concernant l'ultrastructure de Mycobacterium leprae, on a décrit l'ap-parition de membranes symétriques chez des bacillesqui paraissent normaux, provenant d'échantillons fraisou congelés fixés initialement avec des aldéhydes. Dansces études, nous avions admis que des profils symétriques, non retrouvés chez d'autres mycobactéries normales, ne peuvent représenter la structure de lamembrane normale du bacille de la lepre. Dès lors, on a procédé à une nouvelle analyse de l'ultrastructure del't membrane de M. leprae. Dans cette étude, on aétudié la micromorphologie de membranes de M. leprae par microscopie électronique après fixation d'échantillons frais par OsO4 additionné de calcium,et suivi d'une fixation par la glutaraldehyde additionnéde formaldehyde et de calcium, et ensuite par l'uranyl acetate. L'étude des échantillons provenant de deuxmalades soulfrant delépre lépromateuse (LL), de troistabus atteints de lépre naturelle, et d'une souris glabre expérimentalement injectée par la lèpre, a montré queles bacilles d'apparence normale qui sont présents dansles sections colorées au plomb présentaient des mem-branes asymétriques d'une épaisseur de 6, 49 ± 0,36 nanomètres. Ces membranes montraient des constituants qui étaient positifs à l'acide périodique (periodicacid-Schiff PAS): ces constituants étaient exclusivemet situés dans la moitié extérieure de ia double couchede la membrane. On a démontré que le profil symétrique de la membrane de M. leprae décrite dans nos rapports précédents correspondait, comme on l' avait dit dans ces rapports, à une structure anormale de la membrane. Cette anomalie, ainsi qu'on l'a observé, nerésultait pas d'une fixation initiale par des aldéhydes,ou du fait que les échantillons avaient été cntreposéssous forme congelée avant fixation. Ces résultats in-diquent que, malgré qu'elle soit semblable à la membrane des atares mycobactéries d'un point de vue ultrastructural, la membrane de M. leprae a une sensibilité particulière à la fixation par les aldéhycles. Une telle caractéristique, qui n'a pas été retrouvée chez , M. lepraemurium M. aurum, M. avium, et M. tuberculosis H37Ra, doit retléter une structure moléculaire uniquede la membrane, restée inconnue jusqu'à présent.RESUMEN
En reportes previos sobre la ultraestructura de Mycobacterium leprae, describimos la presencia de membranas simétricas en bacilos de apariencia normal, preparados de muestras frescas o congeladas fijadas conzildehídos. En aquellos reportes admitimos que talesperfiles simétricos, los cuales no se encuentran en otras micobacterias normales, no representaban la estructura de la membrana normal del bacilo de la lepra. Enel presente trabajo reanalizamos la ultraestructura dela membrana de M. leprae por microscopía electrónica,después de la fijación de muestras frescas con OsO4 más calcio seguida por glutaraldehído más formalde-hído y calcio seguido por acetato de uranilo. El estudiode las muestras de 2 pacientes con lepra leprornatosa(LL), de tres armadillos con lepra natural, y de un ratóndesnudo con lepra experimental, mostraron que los bacilos de apariencia normal presentes en las secciones teñidas con plomo, tuvieron membranas asimétricas con un grosor de 6.49 ± 0.36 nm. Estas membranas mostraron componentes positivos al ácido peryódicode Schiff localizados exclusivamente en la mitad exterior de la bicapa. En este trabajo demostramos que el perfil simétrico de la membrana de M. leprae descrita en nuestros reportes previos, corresponde a una estructura anormal de la membrana. Ahora se encontróque tal anormalidad es el resultado de Ia fijación primaria con aldehídos o del almacenamiento en congelación de las muestras antes de la fijación. Estos resultados indícan que aunque ultraestructuralmente similar a la de otras micobacterias, la membrana de leprae tiene una peculiar sensibilidad a Ia fijación por aldehídos. Tal característica, la cual no fue encontrada en M. lepramurium, M. aurum, M.avium, y M. tuberculosis H37Ra, debe reflejar una peculiar estructura molecular de la membrana, todavia desconocida.We have reported before on the micromorphology of Mycobacterium leprae in samples from natural and experimental hosts using transmission electron microscopy of ultrathin sections after fixation by a procedure that was found to adequately preserve the ultrastructure of cultivable mycobacteria (9, 11). That procedure consists of a prefixation with glutaraldehyde plus formaldehyde plus calcium, followed by a fixation with OsO4 plus calcium and a postfixation with uranyl acetate (GaFaCa-Os-U). This fixation protocol proved quite useful for the handling of leprosy biopsies because they often have to be sent to a distant electron-microscopy laboratory for processing and aldehyde fixatives are much better suited for this purpose than osmium tetraoxide fixatives. In samples of skin biopsies of lepromatous leprosy patients; the livers, spleens, lymph nodes, and lepromas of armadillos with natural or experimental leprosy; skin biopsies from monkeys with experimental leprosy; and the foot pads of nude mice with experimental leprosy processed for electron microscopy immediately after collection and using the above fixation procedure, continuous membranes of normallooking M. leprae showed a symmetric profile after lead staining and a symmetric Thiéry reaction (21), indicating the presence of periodic acid-Schiff (PAS)-positive components in both membrane layers (7, 11-13,15). Symmetric membranes were also found in M. leprae from fresh skin lepromas from lepromatous leprosy patients after fixation with 4% OsO4 in water supplemented or not with CaCl2(9) and in -70ºC-stored skin lepromas and liver from an armadillo with experimental leprosy after fixation with buffered 1% OsO4 supplemented with several membrane stabilizing divalent cations (10). These observations confirmed the initial finding that no asymmetric membranes could be found in M. leprae, the symmetric profile appearing as the image of the membrane in otherwise normal-looking bacilli.
Such a conclusion, however, raises important questions which were stressed in our previous publications (13, 16). Namely, a) M. leprae membrane symmetry is in contrast with the asymmetry found in all other normal gram-positive bacteria, including mycobacteria and nocardiac, studied until now, which have membranes with the outer layer denser than the inner layer and PAS-positive components exclusively in the outer layer (6, 7, 9, 11, 13), and b) it is difficult to accept, because of well-established concepts on bacterial membrane structure and physiology (7, 23)- that the membrane of normal M. leprae has PAS-positive components in both layers. A symmetric distribution of PAS-positive components in the membrane of M. leprae would represent a striking and unique exception to the concept that the membranes of gram-positive bacteria have amphiphilic molecules, like polysaccharides or equivalent charged polymers, exclusively in the outer layer (7, 23) and, as a consequence, an asymmetric geometry after lead or Thiéry stainings (7). We, therefore, admitted that the symmetric M. leprae membranes seen in the samples we studied would correspond to abnormal membranes (13, 16). The idea that the normal membrane of M. leprae would be asymmetric, like that of other mycobacteria, was strengthened by the recent finding of a few M. leprae cells with asymmetric membranes in a nude mouse with experimental leprosy (16).
We tested the possibility of the abnormal symmetric geometry of the membranes found in otherwise normal-looking M. leprae being the result of a deficient membrane preservation by the GaFaCa-Os-U fixation protocol or of the use of frozen samples for the ultrastructural studies. Such a possibility was confirmed. In the present study, we report in detail the results regarding the detrimental effects of aldehyde prefixation on the ultrastructure of the M. leprae membrane and preliminary data on the alterations induced in that membrane by freezing and thawing.
MATERIALS AND METHODS
Mycobacteria. M. leprae was studied: a) in samples from armadillos 1177,1210, and 1485 collected in southwestern Louisiana, U.S.A., in cooperation with Dr. G. P. Walsh (Armed Forces Institute of Pathology, Washington, D.C., U.S.A.). The animals had naturally acquired leprosy detected by microbiological and histopathological studies carried out by Drs. G. P. Walsh, W. M. Meyers, and C. H. Binford (Armed Forces Institute of Pathology, Washington, D.C., U.S.A.). The samples included skin lepromas, lymph nodes, livers, and spleens; b) in one foot pad from the nude mouse 790-32 with experimental leprosy supplied by Dr. R. C. Hastings (GWL Hansen's Disease Center, Carville, Louisiana, U.S.A.); c) in skin biopsies from two patients with lepromatous leprosy -patient A.F., biopsy supplied by Prof. J. Mesquita Guimarães, Department of Dermatology, School of Medicine, Porto, Portugal, and patient M.S., biopsy supplied by Prof. A. Poiares Batista, Department of Dermatology, School of Medicine, Coimbra, Portugal.
M. lepraemurium (Douglas strain) and M. avium (strain ATCC 25291) were inoculated (2 x 108 bacilli) by the intraperitoneal route into C57BL/6 mice and the livers were collected after 4-5 months. M. aurum (strain 14120005 from the collection of the Institut Pasteur, Paris) was grown in Nutrient Broth (Difco) supplemented with 0.04% Tween 80 at 37º C for 2 days, with occasional shaking.M. avium (same strain as above) and M. tuberculosis H37Ra (strain NCTC 7417) were grown in 7H9 Broth (Difco) supplemented with 0.04% (v/v) Tween 80 at 37ºC, with occasional shaking, for 6 days. As in ocula, exponential cultures in the same medium were used. The bacteria were collected from the broth cultures by centrifugation (2000 x g x 10 min), and the pellets processed for electron microscopy.
Electron microscopy. Two fixation procedures were applied to all samples: a) the previously reported method using aldehyde prefixation (9, 11) [GaFaCa (1 to several days)-OsO4 (16-24 hr)-uranyl (1 hr)]; b) OsO4 (1% in Palade's veronal acetate buffer, pH 6.0, supplemented with 10 mM calcium(4), for 16-24 hr, then washed in several changes of 50 mM cacodylatc buffer supplemented with 10 mM Ca2+, pH 7.0; fixation with GaFaCa (9) for 24 hr; after a wash in water, the samples were postfixed in aqueous 1% uranyl acetate for 1 hr (Os-GaFaCa-U). All fixations were carried out at room temperature. Since we have found that freezing and thawing of M. leprae induces membrane alterations (17), in the present study all samples were fixed within minutes after collection. For comparison, homogenates of the armadillo samples (made in Dubos-Middlebrook liquid medium) were also fixed after one freezingthawing cycle (-70ºC/room temperature). Further processing for electron microscopy was carried out with ethanol dehydration and Epon embedding. Ultrathin sections were cut with an LKB Ultratome III and contrasted with lead citrate (22) for 5 min or stained by the procedure of Thiéry (21) for detection of PAS-positive molecules as modified by us (12). Observations were done with a Siemens' Elmiskop 1A or 102 electron microscope. Tracings of membrane profiles were made on photographic negatives with a Joyce-Loebl MK III CS microdensitometer set to an arm ratio of 50 x and a slit of 0.5 nm.
Terminology of membrane profiles after Thiéry staining. We use here the Thiéry staining method (21) to cytochemically label PAS-positive molecules in ultrathin sections. It has been shown that the Thiéry method is most useful for the detection of the surface amphiphiles in acid-fast bacteria (2, 3, 7, 8, 11-14, 16). Nevertheless, caution is required in the interpretation of the Thiéry labeling seen on bacterial membranes. In brief, we recall now the prerequisites for the accurate interpretation of the technique. A positive Thiéry staining of bacterial membranes consists of the presence of localized black dots; a light staining usually occurs in membrane layers with a negative Thiéry reaction due to the nonspecific staining property of silver proteinates. The membranes of normal gram-positive bacteria give a PAS-positive reaction exclusively in the outer membrane layer, indicating an absolute asymmetry in the distribution of the socalled surface amphiphiles (7, 23). Similarly, in acid-fast organisms the Thiéry stainingof normal membranes reveals the presence of PAS-positive components also restricted to the outer layer (2, 3, 7.8. 11-14). In gram-positive bacteria, including acid-fast bacteria,under degradation both in vitro and in vivo the membrane geometry oflead-stained cells changes from asymmetric to symmetric (6, 7), and the PAS-positive molecules are lost from the outer layer, that is, the membrane becomes PAS-negative (7, 8, 13). In normal bacteria, the membrane profile is always continuous; sometimes, due to undulations in the membrane, the profile may erroneously look interrupted. This must not be confused with true interruptions of the membrane that can occur in bacteria under in vitro or in vivo degradation (6-8).
Three additional membrane patterns are described here for Thiéry-stained mycobacteria membranes primarily fixed with aldehydes: 1) An intermediate pattern characterized by PAS-positive molecules on both membrane layers with predominance in theouter layer; these membranes are difficult to distinguish from the symmetric mem-branes in lead-stained sections. 2) A symmetric pattern characterized by PAS-positive molecules in equal amounts in both membrane layers. This pattern can assume two subtypes: a) Heavy staining of both layers (i.e., the inner layer shows a Thiéry reaction identical to that seen in the outer layer of normal asymmetric-stained profiles); this type of staining is frequently seen in discontinuous membranes. b) Light staining of both layers (i.e., the staining in-tensity of both layers is weaker than that ofthe outer layer of the normal asymmetric membranes); this type of staining is usually found in continuous membranes. In lead-stained sections these two subtypes of membranes appear as symmetric profiles with, respectively, a strong or a light staining ofboth layers.
RESULTS
As is usual with mycobacterial populations in infected hosts (15, 6), normal-looking and degenerating bacilli were found inthe samples studied. In cach sample, the percentage of bacilli with membranes was found to be unaffected by the fixation procedures studied. The quantitative ultrastructural analysis of mycobacterial membranes was, Therefore, carried out by scoringonly bacillary profiles having membranes.
Membrane ultrastructure after immediate fixation. The ultrastructure of the M. leprae membranes in ali samples studied was drastically affected by the fixation conditions used (Table 1). Confirming our previous observations (9-13. 15), after fixation with GaFaCa-Os-U and lead staining almost all bacilli with membranes had symmetric membranes. Many of these symmetric membranes were discontinuous and were present in bacilli with ribosomes (Fig. 1).On the other hand, in samples fixed by Os-GaFaCa-U most of the bacteria with membranes had continuous, asymmetric membranes and ribosomes (Figs. 2a and 2b). The use of the Thiéry staining method showed that the asymmetric membranes in samples fixed with Os-GaFaCa-U had PAS-positive components exclusively located in the outer layer (Figs. 3a and 3b), indicating an absolute asymmetry in the distribution of PAS-positive molecules. In these samples nomembranes were found with a symmetricThiéry staining. In contrast, many symmetric membranes (84.5% of total membranes in armadillo samples) present after fixation with GaFaCa-Os-U showed a strong staining for PAS-positive molecules in both layers; 71.7% of these membranes had gross discontinuities (Fig. 4). It is likely that the actual percentage of bacilli with discontinuous membranes is higher than the value found, because membranes that are continuous in a section may be discontinuous in another plane of sectioning. To clarify this point the study of serial sections is necessary. The use of the Thiéry technique also showed that 3.9% of the bacilli in samples fixed by GaFaCa-Os-U had the intermediate staining pattern.
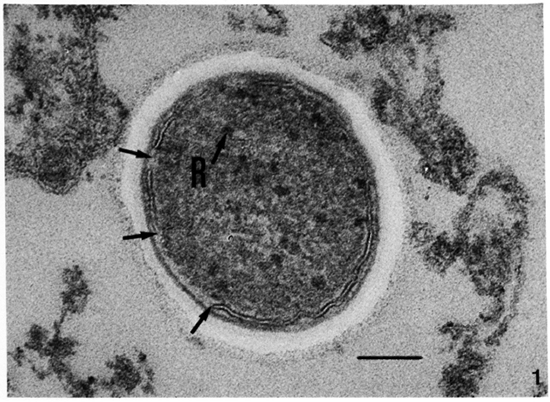
Fig. 1. Ultrastructure of the membrane of M. leprae in a skin leproma from armadillo 1210 after immediate fixation with GaFaCa-Os-U (lead staining). Note symmetric cytoplasmic membrane with discontinuities (unlabeled arrows) and the presence of ribosomes (R). (Bar = 100 nm.)
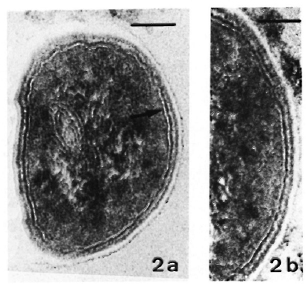
Fig. 2. Ultrastructure of the asymmetric membrane of M. leprae in samples fixed with Os-GaFaCa-U (lead staining). (Bars = 100 nm.)
a = One bacillus from a skin leproma of armadillo 1210. Outer membrane layer is thicker and more electron-dense than inner layer.
b = Sample from nude mouse 790-32. Membrane profile is identical to that in Fig. 2a.
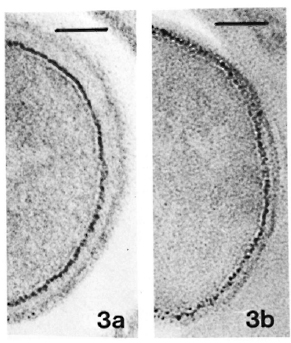
Fig. 3. a = Same sample as in Fig. 2a. Thiéry stain-ing revealing PAS-positive components only in outer layer of the membrane. (Bar = 100 nm.)
b = Same sample as in Fig. 2b. Thiéry staining, showing same pattern of distribution of PAS-positive components as in Fig. 3a. (Bar = 100 nm.)
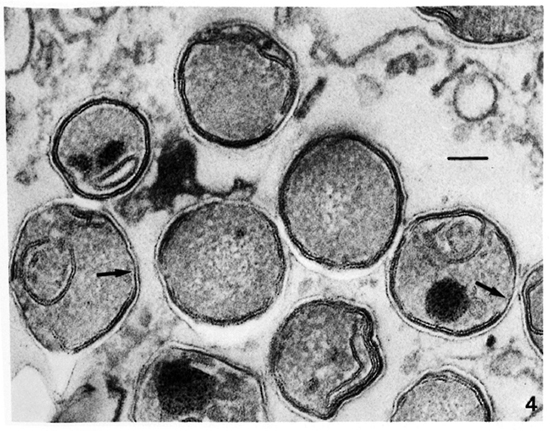
Fig. 4. Thiéry staining of a skin leproma from armadillo 1210, after immediate fixation with GaFaCa-Os-U, showing symmetric membranes with PAS-positive components in both layers. Several membranes have gross discontinuities (unlabeled arrows). (Bar = 100 nm).
The results in Table 1 represent the cumulative values obtained in a quantitative study of samples from armadillos 1210 (skin leproma, liver, and axillary lymph node) and 1177 (tiver). In ali of the samples studied,the above reported patterns of M. leprae membranes in relation to the fixation conditions were observed, indicating that it is a phenomenon common to ali samples studied.
Measurements of the membrane thickness were made on microdensitometric tracings of membrane profiles (Fig. 5a). The mean peak-to-peak distance found for the asymmetric membrane present in lead stained sections of samples fixed with Os-GaFaCa-U was 6.49 ± 0.36 nm (N = 30)(Table 2). This value is significantly lower than that previously reported for the symmetric membranes present in samples primarily fixed by GaFaCa-Os-U (12, 13). This is because the inner layer of the symmetric membranes has PAS-positive components that increase its electron density with the displacement of the inner peak to the cytoplasmic side of the membrane profile (Fig.5b).
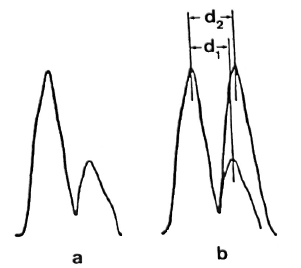
Fig.5. a = Microdensitometric tracing of the membrane of M. leprae fixed with Os-GaFaCa-U and stained with lead. Tracing was made at zone indicatedby the arrow head in Fig. 5a. Outer layer of the membrane is represented by peak on the left; membrane geometry is clearly asymmetric.
b = Asymmetric tracing from Fig. 5a was superimposed on a symmetric tracing constructed by repeating in the inner side of the profile of the outer sidefrom Fig. 5a. Peak-to-peak distance in the asymmetric tracing (d1) is about 10% less than peak-to-peak distance in the symmetric tracing (d2). Such a differenceis identical to the difference between the mean distances found for the asymmetric and symmetric membranes (Table 2). Same result is obtained by super-imposing the asymmetric tracing to a symmetric onefrom membranes fixed with GaFaCa-Os-U.
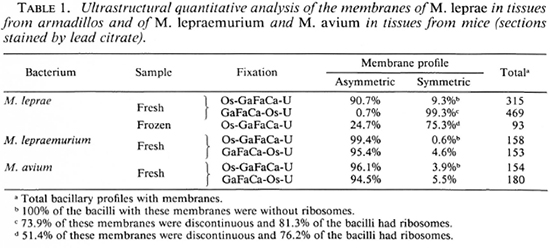
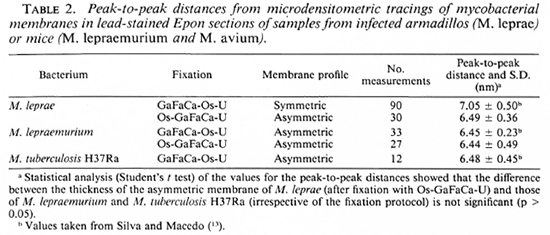
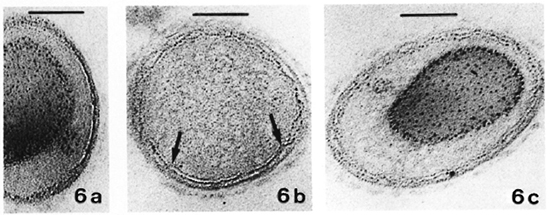
No quantitatively important differences were found in the membrane ultra structure in the in vivo-grown cultivable mycobacteria studied with the two fixation protocols.In Table 1 the results regarding M. leprae-nutriam and M. aviam are presented. Most bacteria have asymmetric membranes with Thiéry-positive components exclusively inthe outer layer (Fig. 6a) regard less of the fixation procedure. In samples fixed by GaFaCa-Os-U, 1.9% and 1.6% of the membranes showed the intermediate Thiéry staining in M. lepraemurium and M. aviam, respectively (Fig. 6b). Moreover, and contrasting with what was found with M. leprae, in M. lepraemurium and M. aviam primarily fixed with aldehydes only 2.0% and 3.8%, respectively, of the membranes showed a symmetric Thiéry staining. In these membranes the intensity of staining of the two membrane layers was lighter than that seen in the outer layer of the asymmetric membranes (Fig. 6c). No discontinuous membranes with this staining pattern were found. In M. lepraemurium and M.avium no membranes were found with astrong Thiéry staining of both layers.
Fig. 6. Thiéry staining of M. avium fixed with GaFaCa-Os-U. (Bar = 100 nm.)
a = Bacillus with a fully asymmetric membrane.
b = Intermediate staining clearly seen at the zone where membrane is cut perpendicularly (between arrows); note inner membrane layer is lightly positive and outer layer has a positive staining that is weaker than that in Fig. 6a.
c = Symmetric, light staining in both membrane layers; note positive reaction is approximately identical in both layers and is lighter than that in outer layer of asymmetric profile in Fig. 6a.
The mean peak-to-peak distance Sound for the asymmetric membranes of M. lepraemurium present in the lead-stained sections of these samples fixed with Os-Ga-FaCa-U was 6.44 ± 0.49 nm (N = 27) (Table2). Similar results were obtained with M.aurum and M. tuberculosis H37Ra in mice,and with in vitro-grown M. avium, M. aurum, and M. tuberculosis H37Ra (not shown). These results confirm previous data showing that fixation with GaFaCa-Os-U is adequate for several cultivable mycobacteria (7, 11).
Ultrastructure of M. leprae membrane after freezing and thawing. We have previously found that freezing and thawing of M.leprae cells induces membrane alterations (17), and a detailed report on this subject is in preparation. For the purpose of the present paper, it suffices to report that after one freezing-thawing cycle the proportion of cells with symmetric membranes seen in lead-stained sections of samples fixed by Os-GaFaCa-U was 75.3% (Table 1); 76.2% of the bacilli with symmetric membranes had ribosomes. The vast majority of those symmetric membranes had PAS-positive components in both layers and 51.4% were frag-mented.
DISCUSSION
Taking into consideration well established concepts on membrane structure and physiology (7, 23) referred to earlier in this paper, we propose that the asymmetric profile of M. leprae membrane now found in fresh samples primarily fixed with buffered and calcium-supplemented osmium tetraoxide is a better morphological expression ofthe membrane structure of the leprosy bacillus than the symmetric profile present insamples primarily fixed by aldehydes. We consider that the two ultrastructural aspects of the membrane of M. leprae seen with the two fixation protocols used in the present study are due to the peculiar sensibility of the membrane to aldehydes, which inducean artifactual symmetric image. This inter-pretation is based on the observation that many M. leprae cells found in samples fixed with GaFaCa-Os-U had symmetric membranes with gross discontinuities but still contained distinct ribosomes; such membranes exhibited PAS-positive components in both layers. The first observation is difficult to reconcile with previous data showing that in gram-positive bacteria under in vitro degradation due to cell lysis or to treatments with membrane-active agents, the membrane alterations are accompanied by ribosome disappearance (5, 18-20) and that mycobacteria degenerating in vivo due to the macrophages' antimicrobial activity have early membrane alterations that are, as well, always accompanied by ribosome disintegration (8). Moreover, Utile symmetric, discontinuous membranes were membranes altered by a degradative process occurringin the host, it is likely that they would have lost their PAS-positive components, as was found with several mycobacteria previously studied (8, 13). The interpretation that the symmetric membranes present in aldehyde prefixed samples are artifactual is further supported by preliminary studies that used a different approach. We have been using the fluorescein diacetate-ethidium bromide staining method of Kvach, et al. (1) in parallel with the quantitative ultrastructural analysis of M. leprae populations in armadillo samples. With the above fluorescence staining, only the bacteria with damaged membranes stain red because the presence of a normal membrane prevents the access of ethidium bromide to the cell interior. We found that in a given sample the percentage of M. leprae cens showing red fluorescenceis much lower than the percentage of cells with membrane ultrastructural abnormalities seen with GaFaCa-Os-U fixation.
The observation that the freezing and thawing of M. leprae induces membrane alterations similar to those produced by aldehyde prefixation (13 and Table 1) explainsour previous finding (1°) of symmetric membranes in M. leprae in samples from skin lepromas and the liver of an armadillo with experimental leprosy that were stored at-70°C before fixation with buffered and calcium-supplemented osmium tetraoxide.
The results now presented confirm our previous contention that the symmetric membranes we found in M. leprae would be abnormal (13, 16 ) and furnish the additional information that such abnormality derives from the fixation technique or from the use of frozen samples.
The membrane of normal M. leprae is not, Therefore, an exception to the general concept that membranes of all normal gram-positive bacteria have PAS-positive components exclusively located in the outer layer (7, 23) and, as a consequence, an asymmetric profile in lead- or Thiéry-stained sections (7). The thickness of the asymmetric membrane now described in M. leprae is not significantly different from that of in vivo-grown M. lepraemurium and M. tuberculosis H37Ra. The present results show, however, that the membrane of the leprosy bacillusis different from the membrane of the other mycobacteria studied in regard to its sensitivity to aldehyde fixatives.
The here-reported occurrence, in M. leprae, M. lepraemurium, and M. avium fixed with GaFaCa-Os-U, of a few membranes with a light Thiéry staining of the inner layer suggests the occurrence of a rearrangement of the membrane structure induced by the aldehydes. Since the membranes with the light Thiéry staining of the inner layer had a lower intensity of staining of the outer layer as compared to the membranes with absolute asymmetry of Thiéry staining, it is likely that the rearrangement of the membrane structure induced by the aldehydes involves the displacement of PAS-positive molecules from the outer to the inner layer. The reported occurrence in M. lepraemurium and M. avium fixed by GaFaCa-Os-U of continuous membranes with a light, symmetric Thiéry staining of both layers is likely to result, as well, from the displacement of PAS-positive molecules from the outer to the inner layer. Therefore, the membranes with PAS-positive molecules in both layers, but with a stronger reaction in the outer layer, would correspond to an intermediate step in the change from fully asymmetric to fully symmetric. In M. leprae, but not in M. lepraemurium and M. avium, the final step in the membrane alteration induced by aldehydes is characterized by the production of discontinuous membranes with a symmetric, strong staining of both layers. This observation suggests that different mechanisms underlie the production of artifactual alterations in the membrane of M. leprae on the one hand, and of M.lepraemurium and M. avium on the other. Morcover, our observations show that the extent of damage imparted to the M. leprae membrane by aldehyde fixatives is by far more important because the alterations are more serious and more widespread.
The few symmetric membranes found in in vivo-grown mycobacteria after fixation with Os-GaFaCa-U probably are membranes altered by degradation by the host's macrophages since they are present in bacteria with out ribosomes and which have lost their PAS-positive components. We have recently shown that change in membrane geometry (with loss of Thiéry-positive molecules) together with ribosome disintegration is an early ultrastructural event in the degradation of mycobacteria by macrophages in vivo (8).
In conclusion, the present results show that, in contrast to the cultivable mycobacteria studied, the agent of human leprosy has membranes that are highly sensitive to aldehyde fixation used routinely for electronmicroscopy. The molecular basis of such apeculiarity remains to be elucidated.
Acknowledgments. The authors are grateful to Dr.Artur Aguas for reading the manuscript and for stimulating discussions, to Dr. C. Resende and Dr. M. Lurdesfor the collection of human skin biopsies, to Dr. Robert C. ilastings for the supply of nucle mouse samples, and to Miss M. Irene Barros for excellent technical assistance.
This work was supported by grants from I.N.I.C.(contract 83/CEN/12), J.N.I.C.T. (contract 897.86.31),Damien Foundation (Brussels), NATO. (grant 470/84) and E.E.C. grant TSD-M-331-B (S).
The Elmiskop 102 electron microscope used in this study was a gift from Stiftung Volkswagen werke over, Federal Republic of Germany).
REFERENCES
1. Kvach, J. T., Munguia, G. and Strand, S. H.Staining tissue-derived Mycobacterium leprae with fluorescein diacetate and ethidium bromide. Int.J. Lepr. 52(1984)176-182.
2. Pettiti'rez, A. and Derieux, J. C. Mis en évidencede polysaccharides sur quelques types de bactéries.J. Microsc. 9(1970)263-272.
3. Robertson, J. G., Lyttleton, P., Williamson ,K. I. and Batt, R. D. The effect of lixation procedures on the electron density of polysaccharide granules in Nocardia corallina. J. Ultrastr. Res. 50(1975)321-332.
4. Ryter, A. and Kellenberger, E. Etude au microscope électronique de plasmas contenant del'acide désoxyribonucléique. I. Les nucléoides des bactéries en croissance active. Z. Naturfor. 13b(1958)597-605.
5. Santos-Mota, J. M., Silva, M. T. and Carvalho-Guerra, F. Ultrastructural and chemicalalterations induced by dicumarol in Streptococcus faecalis. Biochim. Biophys. Acta 249(1971)114-121.
6. Silva, M. T. The ultrastructure of the membrane of grampositive bacteria as influenced by lfxatives and membrane-damaging treatments. In: Bio-menbranes: Lipids, Proteins and Receptors. Burton, R. M. and Packer, L., eds. Webster Groves, Missouri, U.S.A.: Bi-Science Publication Division, 1975, pp. 255-289.
7. Silva, M. T. The use of transmission electron microscopy of ultrathin sections for the characterilation of normal and damaged bacterial membranes. In: Biomenbranes: Dynamics and Biology.Guerra, F. C. and Burton, R. M., eds. New York:Plenum Press, 1984, pp. 1-36.
8. Silva, M. T., Appelberg, R. Silva, M., Nazaré,T. and Macedo, P. M.In vivo killing and degradation of , Mycobacterium aurum by mouse peritoneal macrophages. Infect. Immun. 55(1987)2006-2016.
9. Silva, M. T. and Macedo, P. M. Ultrastructure of Mycobacterium leprae and other acid-fast bacteria as influenced by fixation)conditions. Ann.Nlicrobiol. 133B(1982)59-73.
10. Silva, M. T. and Macedo, P. M. Electron microscopic study of: Mycobacterium leprae membrane.Int. J. Lepr. 51(1983)219-224.
11. Silva, M. T. and Macedo, P. M. The interpretation of the ultrastructure of Mycobacterium cells in transmission electron microscopy of ultrathin sections. Int. J. Lepr. 51(1983)225-234.
12. Silva, M. T. and Macedo, P. M. A comparative ultrastructural study of the membranes of Mycobacterium leprae and of cultivable Myobacteria.Biol. Cell 47(1983)383-386.
13. Silva, M. T. and Macedo. P. M. Ultrastructural characterization of normal and damaged membranes of Mycobacterium leprae and of cultivable, Mycobacteria. J. Gen. Microbiol. 130(1984)369-380.
14. Silva, M. T. and Macedo, P. M. Improved Thiéry staining for the ultrastructural detection of polysaccharides. J. Submicrose. Cytol. 19(1987)677-681.
15. Silva, M. T., Macedo, I'. M., Costa, M. H.,Gonçalves, H., Torgal, J. and David, H. L. Ul-trastructural alterations of mycobacterium /epraein skin biopsies of untreated and treated lepro-matous patients. Ann. Microbiol. 13313(1982)75-92.
16. Silva, M. T., Macedo, P. M., Portaels, F. and Pattyn, S. R. Correlation viability/morphology in Mycobacterium leprae. Acta Leprol.(Genève)95(1985)281-291.
17. Silva, M. T. and Portaels, F. Effects of freezing and thawing on the ultrastructure and viability Mycobacteriam leprae in natural and experimental hosts.(Abstract 129)In: International Symposiumon Mycobacteria of Clinical Interest. Cordoba,1985.
18. Silva, M. T. and Sousa, J. C. F. Ultrastructural alterations induced by moist heat in Bacillus Appl. Microbiol. 24(1972)463-476.
19. Silva, M. T., Sousa, J. C. F., Macedo, M. A. E.,Polonia, J. and Parente, A. M. Effects of phenethyl alcohol on Bacillusl and .Strepiococcus. J. Bacteriol. 127(1976)1359-1369.
20. Silva, M. T., Sousa, J. C. F., Polonia, J. J. and Macedo,P.M. Effects of local anesthetics on bacterial cells. J. Bacteriol. 137(1979)461-468.
21. Thiéry, J. P. Mise én evidence des polysaccharides sur coupes fines en microscopie électronique. J.Microsc. 6(1967)987-1018.
22. Venable, J. H. and Coggeshall, R. A simplified lead citrate stain for use in electron microscopy.J. Cell . Biol. 25(1965)407-408.
23. Wicken, A. J. and Knox, W. W. Bacterial surface amphiphiles. Biochim. Biophys. Acta 504(1980)1-26.
1. M.D., Ph.D.;Research Worker, Centro de Citologia Experimental da Universidade do Porto, Rua do Campo Alegre 823, 4100 Porto, Portugal;
2. Research Worker, Centro de Citologia Experimental da Universidade do Porto, Rua do Campo Alegre 823, 4100 Porto, Portugal;
3. Ph.D., Institute of Tropical Medicine, Antwerp, Belgium.
Received for publication on 25 April 1988.
Accepted for publication on 1 November 1988.