- Volume 69 , Number 2
- Page: 93–8
Vaccination with DNA of the Mycobacterium tuberculosis 85B antigen protects mouse foot pad against infection with M. leprae
ABSTRACT
A DNA vaccine composed of the gene for the common mycobacterial secreted protein antigen 85B was demonstrated to protect the mouse foot pad against infection with Mycobacterium leprae. The protective effect was demonstrated by a 61%-88% reduction in the bacterial number, a protective effect less than that of BCG. The same DNA vaccine has been shown to protect mice against M. tuberculosis infection, and the importance of testing other candidate tuberculosis vaccines for their potential to protect against leprosy is discussed.RÉSUMÉ
Un vaccin à ADN. composé du gène codant pour l'antigène protéinique sécrété commun aux myco- bactéries 85B. a généré une protection contre l'infection de Mycobacterium leprae évaluée par le test d'inoculation à la plante de patte de souris. L'effet protecteur était quantifiable: une réduction de 61 à 88% du nombre de bactéries fut observée, ce qui représente un effet protecteur inférieur à celui généré par le BCO. Ce même vaccin à ADN a montré une protection contre M. tuberculosis, et l'importance de vérifier expérimentalement d'autres candidats de vaccins anti-tuberculaux pour leur potentiel à protéger contre la lèpre, est discutté.RESUMEN
Se demostro que una vacuna de DNA preparada con el gene de la proteína micobacteriana de secreción 85B. protegió al ratón contra la infección por Mycobacterium leprae en el modelo de la almohadilla plantar del ratón. El efecto protector se demostro por una reducción del 61% al 88% en el numero de bactérias, un efecto protector menor al encontrado con BCG.La misma vacuna de DNA protegió al ratón contra la infección por Mycobacterium tuberculosis. Se discute la importancia de probar otros candidates de vacuna contra la tuberculosis, conto inductores poten- ciales de protection contra la lepra.
DNA vaccines are a new approach to vaccination. Plasmids containing genes from a variety of pathogens have been used to induce protection from a variety of bacterial, viral, a protozoal and helminth infections in animal models (7). In mouse models of tuberculosis (TB) and Mycobacterium avium infection, plasmid vaccination has shown an equivalent protective efficacy equal to that of BCG against virulent mycobacterial infection.
The mouse foot pad model of leprosy infection has been used to measure the vaccine potential of various candidate leprosy vaccines over the past 30 years. In this model, a limited self-healing infection is prevented by BCG, live and heat-killed M. leprae (20), various mycobacterial species (21). and leprosy fractions (14,16). This model is the only practicable method for assessing new vaccines for leprosy or for assessing new tuberculosis vaccines for possible cross-protection against leprosy. The mycobacterial antigen 85 (Ag85) family is a group of three closely related proteins that are secreted, bind to (ibronectin and are highly conserved across mycobacterial species (17). Vaccination with the 85B gene protects against tuberculosis infection in the mouse (9). It has been previously demonstrated that immunization of mice with Ag85B DNA was able to elicit a strong immune response. High titers of anti-85B- specific antibody and antigen-speciiic cytotoxic T cells were generated by Ag85B DNA immunization, while antigen-speciiic T-cell proliferation was characterized by the production of interferon-gamma (IFN-γ) and not interleukin-4 (IL-4) (9). The ability of Ag85B DNA to protect against challenge with M. leprae was investigated since vaccination with this vector had protected mice against virulent M. tuberculosis infection (9).
MATERIALS AND METHODS
Production of DNA vaccines. The Ag85B gene was amplified from the M. tuberculosis genome using the ag85b specific primers 5', GTCCGAAGCTTATGACA- GACGTGACGGGA and 3' TAATAG-GATCCTCAGCCGGCGCCTAACGA, and cloned into the vector pJW4303, as previously described (9). The expression of the gene was driven by the cytomegalovirus immediate early promoter with intron A. For immunization, the vector was purified using caesium chloride ultracentrifugation (18) and resuspended in phosphate buffered saline (PBS) at 1 nig/ml. The parental vector pJW4303 was used as the negative control.
Immunization of animals. Swiss albino outbred mice were immunized three times intramuscularly in both hind flanks at 3 weekly intervals with DNA (100 µg per injection). Control mice were immunized three times with PBS or once with 106 of live BCG vaccine intradermally.
Four weeks after the last injections, mice were infected in both hind foot pads with 104 M. leprae in a 30 µl volume. Unvacci- nated mice were inoculated with the same strain to measure the growth of the organisms over 4 to 8 months. Two complete experiments were performed with two strains of M. leprae.
Measurement of M. leprae growth. Foot pads were harvested 6 months after infection with M. leprae and each month thereafter until a growth of 1.5 logs over the inoculum in control mice was reached. The number of bacilli in each foot pad was enumerated by homogenizing the foot pad in 0.1% bovine serum albumin (BSA) in PBS and smearing a calibrated loopful over a slide in an area 8 mm in diameter. The slides were stained by the Ziehl-Nielsen technique. The number of acid-fast bacilli (AFB) in eight l00x oil objective fields was counted and the number of AFB per foot pad was calculated by multiplying the count by the loop volume (l9). The lower level of detection was 10,000 AFB.
RESULTS
Results of the two experiments are shown in Table 1 and in The Figure. Vaccination with control plasmid DNA did not inhibit the growth of M. leprae in the mouse foot pad. By contrast, growth was strongly inhibited in the foot pads of mice vaccinated with either Ag85B DNA or with live BCG. The mean/median AFB counts for each group are shown in Table 1, and show a significant reduction of 61%-88% in bacterial growth. Vaccination with Ag85B DNA was less than the protective efficacy of BCG in the same experiments.
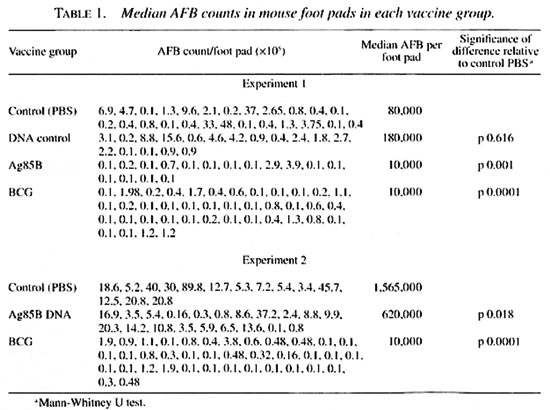
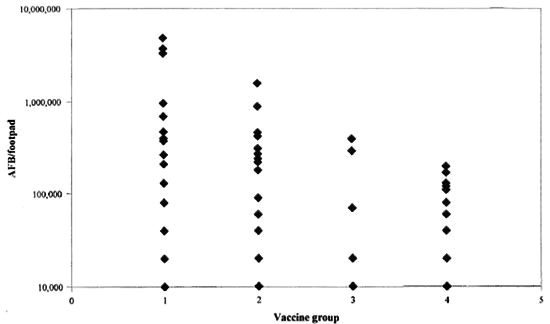
The Figure. Vaccination with DNA protects again M. leprae growth in the mouse foot pad. 1 = Vaccine group 1. unimmunized mice; 2 - group 2, mice immunized with plasmid pJW4303 only; 3 = group 3, mice immunized with 85B gene; 4 = group 4, mice immunized with live BCG.
DISCUSSION
DNA vaccines consist of a circular plasmid with a number of important characteristics which influence the immune potency of the vaccines. The gene of interest is under the control of a strong promoter which drives expression of the protein in host cells. Plasmids persist in muscle cells for long periods and provide a strong, sustained stimulus to the immune system. DNA vaccines containing unmethylated CpG dinu- cleotides within the plasmid are potent adjuvants. DNA vaccines cause polyclonal activation of B cells, macrophages, dendritic cells and a T-helper-1 immune response characterized by cytotoxic T cells and high levels of IFN-γ-producing T cells (10,26). These kinds of immune responses are important in protection from mycobacterial diseases, including leprosy.
This is the second demonstration of a protective effect of DNA vaccines in the mouse foot pad model of leprosy infection. The first demonstration, also from this laboratory (l3), used the gene for the 35-kDa protein in a similar plasmid. The use of the present plasmid has demonstrated that the use of the Ag85B plasmid vaccine has a bidirectional protective efficacy against leprosy and tuberculosis (9). The protection from leprosy infection is equivalent to that obtained by BCG. Our results are in contrast with those of Gillis, et al. (4) who found that vaccination with the closely related Ag85A DNA gave only marginal protection against M. leprae infection in the foot pads of BALB/c mice. The same Ag85A DNA vaccine, however, protected C57/B6 mice against infection with M. tuberculosis (8).
Two reports of DNA vaccines using M. leprae genes to protect against tuberculosis have been published. The MLhsp65 in a transfected macrophage line (22) and as DNA (25) protected against TB challenge. Protection was also achieved with DNA vaccines containing the gene for the M. leprae 36-kDa protein (25). Although hsp65 protein in Freund's complete adjuvant (FCA) protects the mouse foot pad against leprosy infection (6), neither of the DNA vaccines have to our knowledge been tested against leprosy in the mouse foot pad model.
In murine models of tuberculosis infection, a number of DNA vaccines have been tested for protective efficacy. DNA vaccines containing M. tuberculosis genes for the 38-kDa (21), PstS-3 (24), and MTB39A (-) have a protective effect against virulent tuberculosis challenge. However, not all anti-TB DNA vaccines are effective. Vaccines containing genes for the M. tuberculosis 19-kDa lipoprotein (3) and Ag85C (12) fail to provoke T-cell responses to the respective proteins, while vaccination with the DNA of the PstS-1 protein generates a cellular immune response but not protection against virulent challenge (24). Recent work has demonstrated a consistent hierarchy in protective efficacy among DNA vaccines made with three TB protein genes. The present construct was shown to be more effective than vaccines containing the ESAT6 gene which, in turn, was more effective than vaccines containing the MPT64 gene (9). Interestingly, a recent report has shown that DNA vaccines in mouse models of tuberculosis can also have a therapeutic effect, stimulating immune responses in infected animals which can cause the clearance of bacteria from the lungs (11).
While the mouse foot pad model of leprosy infection may provide only an indirect measure of the protective efficacy of these candidate vaccines against human leprosy, the experiments can be done in mouse foot pad laboratories wherever they continue to be active. A review of the results in the mouse foot pad model obtained with various vaccine candidates is presented in Table 2. Although not a disease model, the mouse foot pad infection is prevented by a select range of mycobacteria and some but not all leprosy proteins. As a screening tool, the mouse foot pad model provides us with some important information on the relative importance of different mycobacterial exposures or leprosy proteins in inducing protective immunity. For example, while BCG vaccination prevents infection of the mouse foot pad, vaccination with the M. tuberculosis H37Ra strain does not, despite the close genetic relatedness of these mycobacteria. While immunization with the gene for the Ag85A antigen gave only marginal protection (4), we have demonstrated strong protection induced by the gene for Ag85B, which has more than 75% sequence identity; this points to the specificity of the protective immune response and the utility of the mouse foot pad model to detect differences in the protective efficacy of closely related antigens. The variability in the protective efficacy of the DNA vaccine seen between the two experiments and between individual mice in each experiment is likely due to the variable IFN-γ produced on immunization (13).
New vaccines against tuberculosis are considered a high research priority against the background of the HIV pandemic and outbreaks of multidrug-resistant TB (15). In addition to DNA vaccines, subunit vaccines, recombinant BCG, avirulent mycobacterial vectors and other approaches are being actively pursued. Since there is considerable relatedness between the two causative organisms of tuberculosis and leprosy, any new TB vaccine may have protective efficacy against leprosy as well. With this report, we have established the first, bi-directional DNA vaccine against leprosy and tuberculosis. Now other new candidate TB vaccines should also be examined for leprosy protective effects.
Acknowledgment. The Mycobacterial Research Laboratory is supported by The Leprosy Mission International. The authors gratefully acknowledge Ihe assistance of the staff of the mouse foot pad laboratory.
REFERENCES
1. Baumgart, K. W., McKenzie, K. R., Radford, A. J., Ramshow, 1. and Britton, W. J. Immuno- genicity and protection studies with recombinant mycobacteria and vaccinia vectors coexpressing the 18kDa protein of M. leprae. Infect. Immun. 64(1996)2274-2281.
2. Dillon, D. C., Anderson, M. R., Day, C. H., Lewinsohn, D. M., Coler, R., Bement, T., Cam- pos-Neto, A., Skeiky, Y. A., Orme, I. A., Roberts, A., Steen, S., Dalemans, W., Badaro, R. and Reed, S. G. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 67(1999)2941-2950.
3. Erb, K. J., Kirman, J., Woodfield, L., Wilson, T., Collins, D. M., Watson, J. D. and Legros, G. Identification of potential CD8+ T-cell epitopes of the 19-kDa and ahpc proteins from Mycobacterium tuberculosis- no evidence for CD8+ T-cell priming against identified peptides after DNA- vaccination of mice. Vaccine 16(1998)692-697.
4. Gillis, T. I'., Torerro, M. N., Brennan, P. J., Huygen, K. and Krahenbuhl, J. L. Immune response and protective efficacy of DNA vaccines for leprosy. (Abstract) Int. J. Lepr. 67(1999)492.
5. Gelber, R. H., Brennan, P. J., Hunter, S. W., Munn, M. W., Monson, J. M., Murray. L. P., Siu, P., Tsang, M., Engleman, E. G. and Mo- hagheghpour, N. Effective vaccination of mice against leprosy bacilli with subunits of Mycobacterium leprae. Infect. Immun. 58( 1990)711-718.
6. Gelber, R. J., Mehra, V., Bloom, B. R., Murray, L. P., Siu, P. and Brennan, P. J. Vaccination with pure Mycobacterium leprae proteins inhibits M. leprae multiplication in mouse foot pads. Infect. Immun. 62(1994)4250-4255.
7. Huygen. K. DNA vaccines: application to tuberculosis. Int. J. Tuberc. Lung Dis. 2(1998)971-978.
8. Huygen, K., Content, J., Denis, O., Montgomery, 1). L., Yawman, A. M., Deck, R. R., deWitt, C. M., Orme, I. M., Baldwin, S., D'Souza, C., Drowart, A., Lozes, E., Vandenbussche, P., Van Vooren, J.-P., Liu, M. A. and Ulmer, J. B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nature Medicine 2(1996)893-898.
9. Kamath. A. T., Feng, C. G., Macdonald, M., Briscoe. H. and Britton, W. J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67(1999)1702-1707.
10. Krieo. A. M., Yi, A.-K., Schorr, J. and Davis. H. L. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 6(1998)23-26.
11. Lowrie. D. B., Tascon, R. E., Bonatao, V. I. D., Lima, V. M. F., Faccioli, L. H., Stavropoulos, E., Colston, M. J., Hewinson, R. G., Moelling, K. and Silva. C. L. Therapy of tuberculosis in mice by DNA vaccination. Nature 400(1999)269-271.
12. Lozes, E., Huygen, K., Content, J., Denis. O., Montgomery, D. L., Yawman, A. M., Vandenbusschi:, P., Van Vooren, J. P., Drowart, A., Ulmer. J. B. and Liu, M. A. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding components of the secreted antigen 85 complex. Vaccine 15(1997)830-833.
13. Martin, E., Roche, P. W., Triccas. J. A. and Britton. W. J. DNA encoding a single mycobacterial antigen protects against leprosy infection. Vaccine 19(2001)1391-1396.
14. Ngamying, M., Levy, L. and Brennan, P. J. Vaccination of mice against the leprosy bacillus with skin test antigens. Int. j. Lepr. 67(1999)305-307.
15. Orme, I. Progress in the development of new vaccines against tuberculosis. Int. J. Tuberc. Lung Dis. 1(1997)95-100.
16. Roche, P. W., Neupane. K. D. and Britton, W. J. Cellular immune response to the cell walls of Mycobacterium leprae in leprosy patients and healthy subjects exposed to leprosy. Clin. Exp. Immunol. 89(1992)110-114.
17. Roche, P. W., Peake, P. W., Billman-Jacobe, H., Doran, T. and Britton, W. J. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59. Infect. Immun. 62(1994)5319-5326.
18. Sambrook, .J., Fritsch, E. F. and Maniatis. T. Molecular Cloning: a Laboratory Manual. 2nd edn. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory, 1989.
19. Shepard, C. C. and Mc Rae . D. H. A method for counting acid fast bacteria. Int. J. Lepr. 36(1968)78-82.
20. Shepard, C. C., van Landringham, R. and Walker. L. L. Immunity to Mycobacterium leprae infections in mice stimulated by M. leprae. BCG and graft-versus-host reactions. Infect. Immun. 14(1976)919-928.
21. Shepard, C. C, van Landringham. R. and Walker, L. L. Searches among mycobacterial cultures for antileprosy vaccines. Infcct. I m num. 29(1980)1034-1039.
22. Silva, C. L. and Lowrie, D. B. A single mycobacterial protein (hsp65) expressed by a transgenic antigen presenting cell vaccinates mice against tuberculosis. Immunology 82(1994)244-248.
23. Singh. N. B., Lowe, C. R. E., Rees, R. J. W. and Colston, M. J. Vaccination of mice against Mycobacterium leprae infection. Infect. Immun. 57(1989)653-655.
24. Tanghe, A., Lefevre, P., Denis, O., D'Souza. S., Braibant. M., Lozes, E., Singh, M., Montgomery, D., Content. J. and Huygen. K. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J. Immunol. 162(1999)1113-1119.
25. Tascon, R. E., Colson, M. J., Ragno, S., Stavropoulos, E., Gregory, D. and Lowrie, D. B. Vaccination against tuberculosis by DNA injection. Nature Medicine 2(1996)888-892.
26. Tighe. H., Corr, M., Roman, M. and Raz, E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol. Today 19(1998)89-96.
27. Zhu. X., Venkataprasad. N., Thangaraj, H. S., Hill, M., Singh, M., Ivanyi. J. and Vordermeier, H. M. Functions and specificity of T-cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J. Immunol. 158(1997)5921-5926.
1. Ph.D.; Mycobacterial Research Laboratory, Ananda- ban Leprosy Hospital. Kathmandu, Nepal.
2. Mycobacterial Research Laboratory, Ananda- ban Leprosy Hospital. Kathmandu, Nepal.
3. Mycobacterial Research Laboratory, Ananda- ban Leprosy Hospital. Kathmandu, Nepal.
4. Ph.D. Centenary Institute of Cancer Medicine & Cell Biology, Locked Bag 6. Newtown. NSW. Australia.
5. Ph.D. Centenary Institute of Cancer Medicine & Cell Biology, Locked Bag 6. Newtown. NSW. Australia.
Reprint requests to Dr. Paul Roche. 4 Bendigo St.. 1-isher, ACT. Australia 2611 or e-mail: paulwroche@hotmail.com
Received for publication on 16 October 2000.
Accepted for publication in revised form on 29 March 2001.