- Volume 67 , Number 1
- Page: 24–35
Dermal extracellular matrix in cutaneous leprosy lesions
ABSTRACT
Thirty-eight biopsies of cutaneous lesions f rom leprosy patients [borderline tuberculoid (BT) 14, borderline lepromatous (BL) 18, lepromatous (LL) 6] were processed for staining of some extracellular matrix (ECM) components (collagen, proteoglycans, elastic fibers and fibronectin). Specific histological staining and the indirect immunofluorescence method with antibodies to collagen and fibronectin were utilized. The ECM of the normal dermis was strikingly modified in the inflammatory infiltrate. By Gomori's reticulin and anti-fibronectin immunostaining, replacement of the dense interlaced collagen fibers with a reticular mesh was observed in the infiltrate. The immunoreactivity obtained with anti-type I and anti-type III collagens showed positive fibrils and a lumpy pattern in the lepromatous and tuberculoid lesions with a higher amount in the lepromatous lesions. The lack of clear-cut boundaries between the normal dermis and the inflammatory infiltrate in the lepromatous (BL, LL) lesions was correlated with the blurred limits of the clinical lesions of this pole of the leprosy spectrum. Absence of elastic fibers in the infiltrate was a constant finding, and fuchsin-positive microfibrils were found in some infiltrates. The clear zone of lepromatous lesions was devoid of oxytalan fibers. Elaunin fiber rings around sweat gland acini were present even when the leprosy infiltrate was seen enveloping them. The original ECM is replaced by a newly assembled one, which is suited for the dynamic nature of the inflammatory process. The trophic effects of the ECM upon the cutaneous epithelial structures are modified so that atrophy and late degeneration ensues. These ECM modifications contribute, therefore, to the biological alterations of the skin functions in leprosy.RÉSUMÉ
Trente huit biopsies de lésions cutanées provenant de patients lépreux [14 tuberculoides borderlines (BT). 18 lépromateux borderlines (BL), 6 lépromateux (LL)) furent étudiées pour la coloration des éléments de la matrice extra-cellulaire (MEC) tels que le collagène, les protéoglycannes, les fibres élastiques et la fibronectine. Des colorations spécifiques et la méthode d'immunofluorescence indirecte à partir d'anticorps anti-collagène et anti-fibronectine ont été utilisés pour cette étude. L'infiltrat inflammatoire modifiait de façon frappante la MF.C, telle que présente dans le derme normal. Les fibres denses et entrecroisées de collagène étaient remplacées, dans les infiltrats, par un réseau de fibres visibles par la coloration de la réticuline selon Gomori et positives par immunomarquage spécifique de la fibronectinc. Des fibrilles positives par immunomarquage par des anticorps anti-collagène de type I et de type III, présentaient un aspect en amas irréguliers dans les lésions tant lépromateuses que tuberculoïdes, étant toutefois plus abondantes chez les premières. Il n'y avait pas de limites bien définies entre le derme normal et l'inliltrat inflammatoire dans les lésions lépromateuses (BL, LL), ce qui corrélait bien avec les limites indistinctes des lésions cliniques caractérisant ce pôle du spectre de la lèpre. Il n'y avait pas de fibres élastiques dans l'infiltrat et ce, de façon constante. Des micro-fibrilles positives à la fuchsine furent trouvées dans certains infiltrats. La zone claire des lésions lépromateuses ne présentait pas de fibres oxytalanes. Des anneaux de fibres élaunines autour des acini de glandes sudoripares étaient détectables même lorsque l'infiltrat lépreux les enveloppait complètement. La MEC pré-existante est remplaçée par une matrice néoformée, qui est adaptée au caractère très dynamique du processus inflammatoire. Les actions trophiques de la MEC sur les structures cutanées épithéliales sont modifiées de telle manière qu'il s'ensuit atrophie et dégénération de ces dernières. Ces modifications de la MEC contribuent de ce fait à l'altération des fonctions cutanées observées au cours de la lèpre.RESUMEN
Se proccsaron 38 biópsias de lesiones cutâneas de pacientes con lepra (14 con lepra luberculoide subpolar, BT; 18 con lepra lepromatosa subpolar, BL; 6 con lepra lepromatosa, LL) para la tinción de algunos componentes de la matriz extracelular (CME): colágena, proteoglicanas, libras elásticas y libronectina. Sc utilizaron tinciones histológicas específicas y la inmunoluorescencia indirecta con anticuerpos contra colágena y libronectina. Los CME de la dermis normal resultaron marcademente modificados en el infiltrado inflamatório. La tinción para reticulina de Gontori y la inmunotinción para libronectina, revelaron en el infiltrado la substitución de las fibras de colágena densamente entrelazadas, por una trama de libras reticulares. La inmunoreactividad obtenida con anticuerpos anti-colágena tipo I y colágene tipo III mostro fibrillas positivas y un patron moteado en las lesiones lepromatosas y tuberculoides, con predomínio en las lesiones lepromatosas. La falta de limites definidos entre la dermis normal y el infiltrado inflamatório en las lesiones lepromatosas (BL y LL) correlaciono con los limites difusos de las lesiones clínicas observadas en este polod de Ia enfermedad. La ausência de fibras elásticas en el infiltrado fue un hallazgo constante; en algunos infiltrados se encontraron microfibrillas fuchsina- positivas. La zone clara de las lesiones lepromatosas estuvo libre de fibras de oxitalan. Los anillos de fibras de elaunina alrededor de los acini de las glândulas sudoríparas fueron evidentes aun cuando estuvieron envueltos por los infiltrados de la lepra. Los câmbios en los componentes de la matriz extracelular son indicativos de lo dinâmico dei proceso inflamatório. Los electos tróficos de los CME sobre las estructuras de los epitelios cutâneos son de tal magnitud que provocan Ia atrofia y la degeneración tardia de la piei. Estas modificaciones en los CME también contribuyen a las alteraciones en las functiones de Ia piei en la lepra.The extracellular matrix (ECM) has been considered an environment of crucial importance, influencing cell behavior. It can work as an adhesion substrate for migrating cells (6, 17, 26) or for released growth factors (13). The ECM also functions as costimulatory signals for cell proliferation and differentiation (12).
The function of cell populations in the tissues can be inferred through the analysis of their extracellular matrix. Development, neoplasia and inflammation have common features related to cell-matrix interaction. Therefore, there is an intimate relationship between the assembly of the tissue extracellular matrix and the biological activity. The knowledge from research on cell-matrix interactions in development and in oncogenesis may be applied to the interpretation of the inflammatory process.
Experimental models of murine schistosomiasis show granulomas with remodelling of the original hepatic extracellular matrix and with a stage-dependent preferential accumulation of collagen (type I and type III) (28, 30)), proteoglycans (dermatan-sulfate and heparan sulfate) (5), and fibronectin during the formation of the granulomatous structure (16). A concomitant proteolytic activity represented by collagenase is also detected (39). Maximal degradation and synthesis coincides with the peak of the mouse immune response to the presence of Schistosoma mansoni ova in the liver, linking the matrix remodelling occurring in granulomas to immunological factors (39).
ECM changes in leprosy have been studied, emphasizing the presence of auto-antibodies to collagen in the serum of lepromatous patients (25), the pattern of fibronectin in different polar granulomas (26) and the presence of anti-elastic microfibril antibodies in the sera of patients with leprosy (24).
In this work, we studied the effects of leprosy inflammatory lesions upon the dermal ECM components (collagen, proteoglycans, elastic fibers and fibronectin), correlating the findings with the corresponding patient's clinical and histopathological picture according to the Ridley-Jopling classification (35). An overview of dermal ECM structural changes in leprosy lesions will raise some specific questions for each matrix component involved. Furthermore, pathogenetic implications can be drawn from the global comprehension of the altered cell-matrix interaction in the leprosy lesions.
MATERIALS AND METHODS
The study was done with cutaneous lesion biopsies from 38 leprosy patients from Souza Araújo Ambulatory (Laboratory of Leprosy), Department of Tropical Medicine (Instituto Oswaldo Cruz), Rio de Janeiro, Brazil. The patients were grouped according to the Ridley-Jopling classification (35) and were distrusted among the leprosy spectrum as follows: borderline tuberculoid (BT) 14, borderline lepromatous (BL) 18, lepromatous (LL) 6. Biopsy specimens of the lesions were cut in half; one half went through routine paraffin embedding, the other half was snap-frozen in liquid nitrogen. Paraffin sections were stained with hematoxylin and eosin (H&E) and Wade (for acid-fast bacilli) staining; picrosirius (PS) (19), phosphomolybdie acid-picrosirius red (PMA-PSR) (8), Gomori's reticulin (GR) (15) for collagen and reticular fibers, respectively; periodic acid Schiff-alcian blue at pH 1 (PAS-AB pH 1.0) and at pH 2.5 (PAS-AB pH 2.5) (37) for sulfated and non-sulfated proteoglycans, respectively; resorcin-fuchsin (RF) (4) for elastic fibers.
Five-µm-thick cryostat sections were thawed onto glass slides, fixed in acetone at 4°C for 20 min, dried at room temperature for 5 min, and submitted to the following staining methods: PAS AB pH 1 and 2.5, resorcin-fuchsin staining and indirect immunofluorescence (IIF) (3) for collagens (type 1 and III), fibronectin and tenascin. Incubation of the sections with polyclonal anti-human collagen I (diluted 1:40), rabbit antihuman collagen type III (diluted 1:40) and rabbit anti-fibronectin antibody (diluted 1:50) was followed by a fluorescein-conjugated goat anti-rabbit immunoglobulin second antibody (diluted 1:50) (DAKO, Glostrup, Denmark) or fluorescein-conjugated goat anti- mouse immunoglobulin (DAKO). Counter-staining with 1% Evans blue (Merk, Germany) for 10 min was done. The slides were mounted with buffered glycerin to which 1% p-phenylenediamine (33) (Sigma Chemical Co., St. Louis, Missouri, U.S.A) was added. The secondary antibody was controlled by omitting the primary one and the primary one by incubating the sections with antibodies of unrelated specificity (anti-Factor VHI-related antigen).
The specimens were examined under a Carl Zeiss photomicroscope (Zeiss, Germany) and for confocal microscopy on a laser scanning microscope (LSM) 410 inverted (Zeiss) with planapochromatic x40, 1.3 NA, oil immersion objective. Antibodies labeled with fluorescein were read on an argon/krypton-ion-laser at 488 nm, pinhole set in aperture 20 with a BP 510-525 emission filter. PMA-PSR was examined with the same Iaster at 543 nm with an LP 570 emission filter. Images were obtained using slow scanning and line Kalman averaging. The confocal scanning optical microscopy was not applied to generate three-dimensional (3D) images but to remove out-of-focus blur from non-confocal fluorescent microscopy. This instrument, due to the confocal principle, allows optical tomography (optical sections without touching the object) information collection only out of one plane and 3-D reconstruction of the collected sections (38). The original digital images were printed on a Codonics NT 1600 printer.
RESULTS
Remarkable alterations of the dermal ECM in the leprosy infiltrates were observed (The Table). The normal interlacing arid thick dermal collagen bundles were completely modified in both tuberculoid and lepromatous lesions (Figs. 1A, IB; 2A, 2B). Normal dermal collagen septa remained among the strings or foci of the inflammatory infiltrate in the tuberculoid lesions (Fig. 1A). Phosphomolybdic acid-picrosirius red staining analyzed by confocal microscopy developed a delicate network of collagen fibers in lepromatous lesions and an absence of fibers in the tuberculoid ones (Figs. IB, 1C). By immunofluorescence with confocal microscopy, type I and III collagen fibers were also more evident in the lepromatous lesions and almost absent in areas occupied by epithelioid cells (Figs. 2A, 2B). A lumpy pattern of collagen immunoreactivity was also observed in the lesions, mainly in the conventional immunofluorescence studies (Fig. 3A). The dermal clear zone of the lepromatous pole of the disease spectrum showed a parallel-to-epidermis fiber arrangement (Fig. 2B). Go- mori's reticulin staining displayed a dense irregular reticular mesh in both tuberculoid and lepromatous lesions with no distinguishing characteristics between the two groups (Figs. 4A, 4B). The reticular net observed in the lesions was not similar to the PS-stained one; however, an architectural similarity of the fibronectin-immunoreactive fibers and the silver-impregnated ones was observed in the lesions of both the tuberculoid and lepromatous poles (Figs. 5A, 5B). Clear-cut boundaries between the tuberculoid infiltrates and the normal dermis were observed with the PMA-PSR (Fig. 1 A); whereas in the polar lepromatous, the limits of the normal matricial dermis with the matrix of the infiltrate were blurred (Fig. 1B). This was due to the intermingling of the newly assembled inflammatory matrix in the periphery of the infiltrate with that of the normal dermis.
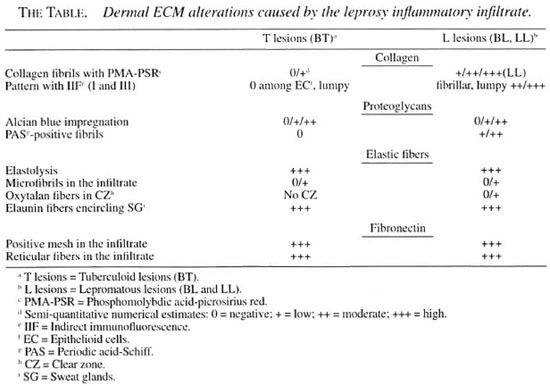
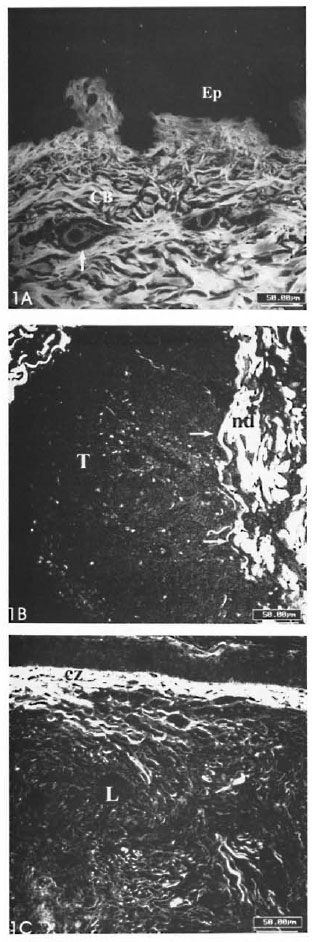
Fig. 1. A = Section of normal skin. Compact and interlacing collagen liber bundles (CB) in the dermis are seen.↑= A looser matrix with line collagen librils is shown surrounding the upper dermal vessels; Ep = epidermis. (PMA-PS). B = Complete absence of collagen libers in a borderline tuberculoid lesion (T). Remaining septa of normal dermis (nd) are seen. →→ = Boundaries between lesion and normal dermis are clear-cut. C = Fine collagen libers in a lepromatous polar lesion (L). Parallel-to-epidermis liber arrangement is observed in the clear zone (cz) (PMA-PSR; confocal microscopy; scale bars: 50 µm).
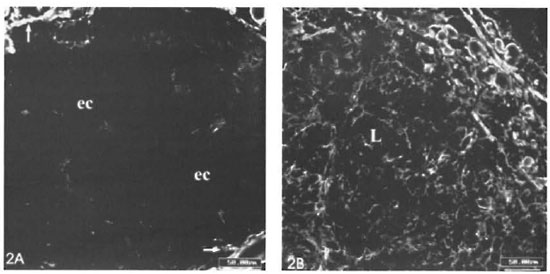
Fig. 2. A = Few type III collagen fibers in a tuberculoid infiltrate. Dark central region of the figure is negative for anti-collagen III and is occupied by epithelioid cells (ec).↑ = Remaining septa of normal dermis can be observed in the corners of the figure. B = Shows plenty of fine collagen III-immunoreactive fibers in a lepromatous lesion (L) (Confocal microscopy; scale bars: 50 µm).
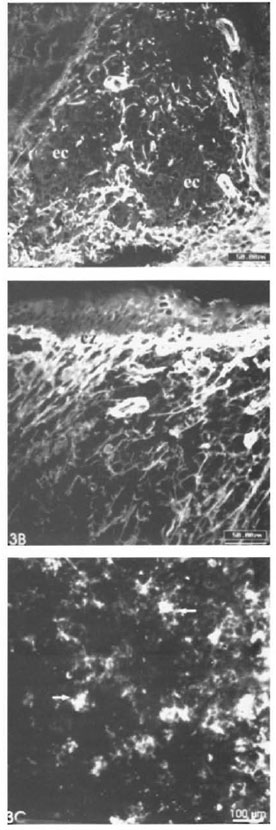
Fig. 3. A = Few collagen type I-positive libers in the central region of a tuberculoid granuloma occupied by epithelioid cells (ec) (scale bar: 50 µm). B = Large amount of line collagen I libers in a polar lepromatous infiltrate. Clear zone (cz) is strongly stained (scale bar: 50 µm). C = Lumpy-starry pattern (→) in a lepromatous lesion (IIF; scale bar: 100 µm).

Fig. 4. A = Silver impregnation (Gomori's reticulin) of normal skin section. Reticular libers are only observed in the regions surrounding vessels (scale bar: 100 µm). B = Reticular liber mesh in a tuberculoid lesion (T), showing sparse density among central epithelioid cells (ec). Fibrous septae were formed among expanded granulomas. C = Diffuse reticular liber mesh in a lepromatous lesion (L) (Gomori's reticulin; scale bars: 100 µm).
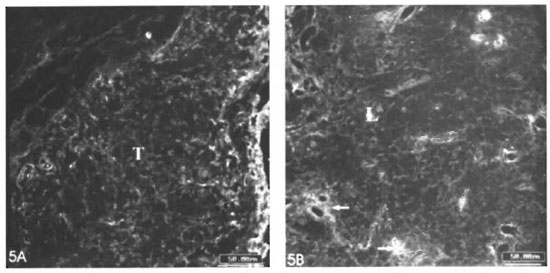
Fig. 5. A = Fibroncclin mesh in a tuberculoid lesion (T). B = Fibronectin mesh in a lepromatous lesion (L). An intensification of the inimunoreaetivity is observed in the regions surrounding vessels (IIF; photomicrography; scale bars: 50µm).
The diffuse alcian blue impregnation, which is normally observed in the dermis due to its proteoglycan content, was substituted in the granuloma for a faintly (pH 1.0) alcianophilic material embedding the inflammatory cells of the epithelioid infiltrate. PAS-positive fibrillar material was seen among cells in the polar lepromatous lesions but not in the epithelioid infiltrates (Fig. 6A). The luminal surface of the endothelial cells lining the internal microvessel wall was alcian blue-positive.
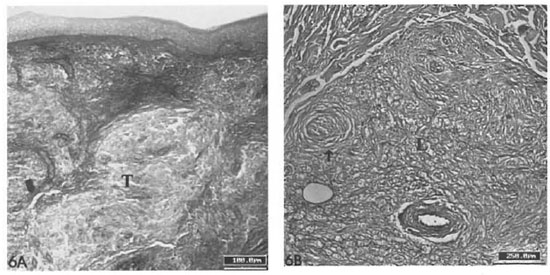
Fig. 6. A = Absence of PAS-positive fibrils in epithelioid granulomas of borderline tuberculoid lesion (T) (scale bar: 100 µm). B = Presence of PAS-positive librils in a lepromatous lesion (L). A peripheral nerve branch (→) with hyperplastic perineurium is seen (PAS-AB phI 1.0; scale bar: 100 µm).
The elastic fibers were constantly absent in both lepromatous and tuberculoid lesions (Figs. 7A, 7B). Short and thin fuchsin- stained microfibrils were observed in the leprosy infiltrate of lepromatous lesions (BL, LL) in the frozen sections. The oxytalan and elaunin fibers in the dermal clear zone were fragmented or had disappeared completely (Fig. 7B); this finding was associated with an atrophic epidermis over the clear zone. The elaunin fiber rings surrounding the sweat gland tubules were observed even when the leprosy inflammatory infiltrate involved these adnexal structures.
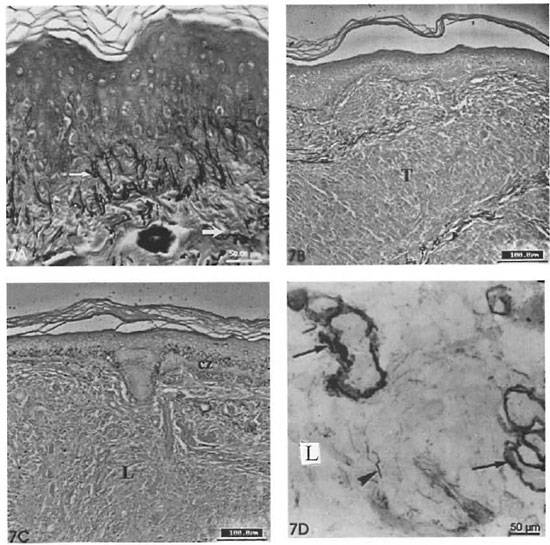
Fig. 7. A = Section of normal skin stained with resorcin-fuchsin. Note fine oxytalan libers (→) in papillar dermis and thick mature elastic libers in the dermis (→) (scale bar: 50 µm). B = Absence of elastic libers in a tuberculoid lesion (T) (scale bar: 100 µm). C = Absence of elastic libers in lepromatous lesion (L). Oxytalan libers are absent in the clear zone (cz) (RF; scale bar: 100 µm). D = Close view of fuchsin-stained microfibrils in a lepromatous infiltrate ( ) and elaunin libers wrapping sweat gland acini (→) (scale bar: 50 µm).
) and elaunin libers wrapping sweat gland acini (→) (scale bar: 50 µm).
A dense and diffuse fibronectin mesh was detected in tuberculoid and lepromatous lesions with intensification of the im- munoreactivity around the inflammatory microvessels (Figs. 5A, 5B). As already mentioned, this mesh was very similar to the one formed by the reticular fibers. The fibronectin distribution remained unaltered in the unaffected dermis.
DISCUSSION
Investigation of the inflammatory ECM alterations coupled with research on the participating cell populations in leprosy provide an integrated comprehension of the inflammatory process in this disease. Vascular, epithelial and ECM structures participate actively in inflammation, driving leukocyte migration (7), interacting with leukocytes or secreting stimulatory cytokines (keratinocyte) and functioning as adhesion substrate (27) or as costimulatory signals (2) (ECM). The basic finding of this work is the replacement of the normal compact ECM of the dermis for a looser matrix in the sites occupied by granulomas. This newly assembled matrix is one of the expanding-sustaining type and suited to the dynamic inflammatory process (12-21). According to Gailit and Clark (12) and to Gospodarowicz, et al . (14), a loose matrix is more appropriate for the inflammatory environment where cellular turnover is very high and where plenty of mediators and cytokines must be provided to the inflammatory cells. This new substrate is also suited for cell adhesion as well. The substitution of the original matrix is mainly performed by means of collagenolysis, proteoglycan degradation, elastolysis and collagen neosyn- thesis. The following factors are known to contribute to the formation of this new ECM assembly; a) collagenase and elastase produced by macrophages and other inflammatory leukocytes are responsible for the removal of the original matrix components (39); b) fibronectin deposition by means of plasma extravasation and production by fibroblasts turns this provisional substrate (16) into one proper for mononuclear cell invasion (9) and c) collagen production stimulated by fibrogenic cytokines produced by macrophages and lymphocytes are also involved in the process (11). The amount of collagen in inflammatory lesions depends, thus, on the balance between fibrogenic and fibrolytic cytokines. Interleukin-1 (IL-1 ) stimulates fibrogenesis; whereas gamma-interferon (IFN-y) inhibits this process (11). In the case of leprosy infiltrates, the decreased IFN-y production by lymphocytes from lepromatous patients (29) could be, in a speculative way, the cause for the larger amounts of collagen deposition in the lepromatous lesions found in this work.
The lumpy collagen-immunoreactive pattern found in the leprosy infiltrates was probably due to local production of collagen adjacent to the fibroblasts which sometimes acquire a star-like shape (Fig. 3C).
The distinct morphological boundaries between the normal and the inflammatory matrix (clear-cut in tuberculoid and blurred in lepromatous infiltrates) may correspond to the clinical aspect of the lesions which can either present borders neatly outlined or blurred, respectively (1). Since it is more collagenolytic and more fibrogenic, the border of a lepromatous lesion is usually not outlined because the newly assembled matrix is intermingled with the surrounding matrix of the normal dermis. Tuberculoid plaques, however, are less fibrogenic in the central region occupied by the epithelioid cells (Figs. IB, 2A, 3A), keeping their outer limits neat. The lower amounts of collagen fibers observed in the tuberculoid infiltrates may occur due to the inhibition of collagen fibril formation, by unknown mechanisms, in the scarce intercellular space among epithelioid macrophages.
The absence of elastic fibers is explained by the release of elastase by inflammatory leukocytes, mainly macrophages. On the other hand, products of elastin degradation are chemoattractants for fibroblasts (6). Elastic fibers have a microfibrillar core and an amorphous elastin component which is distributed among the 6-nm-thick microfibrils. There are normally three types of elastic fibers: oxytalan, elaunin and mature elastic fibers. The three types of fibers differ one from the other only by the amount of elastin. This protein is absent in oxytalan fibers, scarce in elaunin and fully present in mature fibers. Oxytalan fibers are only present in the papillar dermis and elaunin fibers can be observed surrounding the sweat gland acini (10). Elastic fibers function almost exclusively as a mechanical support and are promptly removed in the inflammatory infiltrate (10). Their repair is usually not performed in the inflammatory lesions.
We saw, in frozen sections, small fibrils which looked like oxytalan fibers in the lesions, similar to what was observed in Schistosoma mansoni granuloma by Cotta-Pereira, et al. (4). It is impossible to determine whether these small fibrils are residual microfibrils after elastin digestion or if they are a morphological expression of elastic fiber repair. Sequential morphological studies with ultrastructural methods could be useful to clarify this question. The absence or fragmentation of oxytalan and elaunin fibers in the clear zone of lepromatous lesions may probably either be caused by the pushing and expanding lepromatous infiltrate or by the epidermal atrophy that is usually associated with these lesions. The epidermis probably influences the production of the oxytalan fibers, since in the skin they are only present at the dermo-epidermal junction. The elaunin fiber rings enveloping sweat gland tubules were preserved in spite of being affected by advanced leprosy inflammatory infiltrate. This indicates that the inflammatory infiltrate does not cause direct injury to these adnexal structures. Inflammatory cells do not infiltrate the adnexal epithelium but they modify the periadnexal ECM and change matrix-epithelial interactions, resulting in degeneration of adnexal structures.
The fibronectin mesh found in the lesions of both lepromatous and tuberculoid infiltrates have similar appearances. The fibronectin immunoreactive pattern of both tuberculoid and lepromatous lesions are similar to the silver-impregnated reticular mesh. No difference was observed in the reticular architectural pattern in the tuberculoid and lepromatous lesions as well, except that reticular libers were scarce among the central epithelioid cells of the tuberculoid granulomas (20). According to Wolman and Kasten (40), fibronectin could be a constituent of the reticular fibers. PAS-positive fibrillar material in the more organized polar lepromatous lesions may also correspond to fibronectin inflammatory mesh. Fibronectin is a multifunctional component of the loose connective tissue suited for the dynamic interactions of inflammation. It is also associated with opsonization (31, 34 ), and with cell-matrix interaction by means of integrin mediation (18). Mycobacterium leprae presents a fibronectin-attachment protein (FAP) which is involved in the internalization of the bacteria by Schwann cells and epithelial cells (36). Antibodies to the fibronectin binding 85 kDa ABC antigenic complex of M. leprae were found to be raised in lepromatous sera(32). This antigen complex can also elicit a Till response on the lymphocytes of leprosy contacts Patti and Hook (31) have used the acronym MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) to identify the family of adhesins which recognize and bind amino-acid sequences of matrix components. Diverse bacterial tissue tropism and virulence can occur because of specific MSCRAMMs.
In addition, the incidence of reactional episodes or changes in the activity of the leprosy lesion could, in a speculative way, be associated with changes in either the fibronectin or proteoglycan composition of the inflammatory matrix. These two components are closely associated with haptotaxis and cell migration, respectively, which undoubtedly occur in the early stages of leprosy reaction.
The remarkable ECM alterations seen in cutaneous leprosy lesions involve collagen, proteoglycans, elastic fibers and fibronectin. Although distinct cell populations are observed in the inflammatory infiltrates corresponding to the different forms of disease presentation, no striking differences on ECM components were found between the tuberculoid and lepromatous poles inside the lesions. Therefore, ECM cannot be an additional aid parameter in the classification of leprosy. The striking ECM modifications caused by the leprosy inflammatory process affect the biological properties of the skin and, additionally, contribute to the clinical manifestations of the disease.
REFERENCES
1. Bkyckson, A. and Pfaltzgraff, R. F. Symptoms and signs. In: Leprosy . Edinburgh: Churchill Livingstone, 1990, pp. 25-56.
2. Chang, A. C., Salomon, D. R., Waixsworth, S., Hong, M. P., Mojcik, C. F., Otto, S., Sherach, E. M. and Coligan, J. E. α3 β1 α β1 integrins mediate laminin/merosin binding and function as costimulatory molecules for human thymocyte proliferation. J. Immunol. 154(1995)500-510.
3. Coons, A. H. Fluorescent antibody methods. In: General Cytochemical Methods, Vol. 1 . Danielli, J. F, ed. New York: Academic Press, 1958, pp. 399-422.
4. Cotta-Phreira, G., Len/.i, H., Oliveir v, D. N., Wajsenzoni, J. R. and Lenzi, J. A. On the presence of elastic microfibrils in liver granuloma of murine Schistosomiasis mansoni . Mem. Inst. Oswaldo Cruz 86 Suppl. III(1991)129-130.
5. Da Silva, L. C., Mourâo, P. A. and Borojevic, R. Patterns of sulfated glycosaminoglycan synthesis and accumulation in hepatic granulomas induced by schistosomal infection. Exp. Mol. Pathol. 50(1989)411-420.
6. Davidson, J. M. and Giro, M. G. Control of elastin synthesis. In: Regulation of Matrix Accumulation . Orlando: Academic Press, Inc., 1986. pp. 178-217.
7. Degos, L. and Kahn, A. Adhésion cellulaire. Med. Sci. 3(1987)314-315.
8. Dolber, P. C. and Spach, M. S. Conventional and confoeal fluorescence microscopy of collagen fibers in the heart. J. llistochem. Cytochem. 41(1993)465-469.
9. Dvorak, H. F. Tumor wounds that do not heal. N. Engl. J. Med. 315(1986)1650-1659.
10. Franzblau, C. and Faris, B. Elastin. In: Cell Biology of the Extracellular Matrix . Hay, E. D., ed. New York, Plenum Press. 1981. p. 65.
11. Freundlich, B., Bowailaski, J. S., Nelson. E. and Jimenez, S. A. Regulation of fibroblast proliferation and collagen synthesis by cytokines. Immunol. Today 7(1986)303-307.
12. Gailit, J. and Clark, R. A. F. Wound repair in the context of extracellular matrix. Curr. Opin. Cell Biol. 6(1994)717-725.
13. Gordon, M. Y, Rilky, G. P.. Wait, S. M. and Greaves, M. F. Compartmentalization of a hemopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature 326(1987)403-405.
14. Gospodarowicz, D., Gri i nhhuc., G. and Bird- well, C. R. Determination of cellular shape by extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 38(1978)4155-4171.
15. Gridley, M. F. Stains for connective tissue. In: Manual of Histologic ami Special Staining Technics . New York: McGraw Hill, 1960, pp. 55-96.
16. Grimaud, J. A., Boros, D. L., Takiya, C., Mathew, R. C. and Emonard, H. Collagen isotypes, laminin, and fibronectin in granulomas of the liver and intestines of Schistosoma mansoni -infected mice. Am. J. Trop. Med. Hyg. 37(1987)335-344.
17. Hay, E. D. Collagen and embryonic development. In: Cell Biology of Extracellular Matrix . Hay, E. D., ed. New York: Plenum Press, 1982, pp. 379-410.
18. Hynes, R. O. Integrins: a family of cell surface receptors. Cell 48(1987)549-554.
19. Junqueira, L. C. U.. Bignolas, and Brentam, R. R. Picrossirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 11(1979)447-455.
20. Khanolkar, V. R. Pathology in leprosy. In: Leprosy in Theory and Practice . Cochrane, R. G., and Davey, T. F., eds. Bristol: John Wright, 1964, pp. 125-151.
21. Klein, G. The extracellular matrix of the hematopoietic microenvironment. Experientia 51(1995)914-926.
22. Launois, P., N'Diaye, M. N., Cartel, J. L., Mane, I., Drovvart, A.. Van Vooren. J. P., Sarthou, J. L. and Huygen, K. Fibronectin-binding antigen 85 and the 10-kilodalton GroES-related heat shock protein are the predominant Th-1 response inducers in leprosy contacts. Infect. Immun. 63(1995)88-93.
23. Lenzi, H. L.. Calich, V. L. G., Miyaji, M., Sano, A., Nishimura, K. and Burger, E. Fibrosis patterns of lesions developed by athymic and euthymic mice infected with Paracocciclitxles brasiliensis . Braz. Med. Biol. Res. 27(1994)2301-2308.
24. Linder, E., Lehto, V. P., Stenman, S., Lindkvist, K., Bjorvatn, B. and Bergkvist, R. Circulating antibodies to connective tissue microfibrils and dermal immunoglobulin deposits in leprosy. Clin. Immunol. Immunopathol. 13(1979)1-8.
25. McAdam, K. P. W. J., Fudenherg, H. H. and Michaeli, D. Antibodies to collagen in patients with leprosy. Clin. Immunol. Immunopathol. 9(1978)16-21.
26. Narayanan, R. B., Bhutani, L. K., Sharma, A. K. and Nathi, I Fibronectin in leprosy lesions; observations using monoclonal antibodies to human fibronectin. Indian J. Lepr. 56(1984)532-539.
27. Newgrehn, D. The role of the extracellular matrix in the control of neural crest cell migration. In: Extracellular Matrix . Hawkes, S. and Wang, J. L., eds. New York: Academic Press, 1982. pp. 141-146.
28. Nishimura, M.. Asahi M.. Hayashi, M., Takazono, I , Tanaka. Y., Kohda, H. and Urame, H. Extracellular matrix in hepatic granulomas of mice infected with Schistosoma mansoni : qualitative and quantitative analysis. Arch. Pathol. Lab. Med. 109(1985)813-818.
29. Nogueira. N.. Kaplan, G., Levy, E., Sarno, E. N., Kusiiner, I., Granelli-Piperno, A.. Vieira, L., Gould, V. C., Li a is. W., Steinmann, R., Yip, Y. K. and Cohn, Z. A. Defective y-interferon production in leprosy. J. Exp. Med. 158(1983)2165-2170.
30. Olds, G. R., Griffin, A. and Kresina, T. F. Dynamics of collagen accumulations and polymorphism in murine Schistosoma japonicum . Gastroenterology 89(1985)617-662.
31. Patti. J. M. and Hook, M. Microbial adhesins recognizing extracellular matrix macromolecules. Curr. Opin. Cell Biol. 6(1994)752-758.
32. Pessolani, M. C.. Peralta, J. M., Rumanek, F. D., Gomes, H. M.. Marques, M. A.. Almeida, E. C., Saad, M. H. and Sarno, E. N. Serology and leprosy: immunoassays comparing immunoglobulin G antibody responses to 28- and 30-kilodalton proteins purified from Mycobacterium bovis BCG. J. Clin. Microbiol. 29(1991)2285-2290.
33. Platt, J. L. and Michael, A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenilenediamine. J. Histochem. Cytochem. 157(1983)1339-1353.
34. Proctor. R. A.. Prlndergast, E. and Mosher, K. F. Opsonization of bacteria by fibronectin. Blood 59(1982)651-687.
35. Ridley. D. S. and Jopi.ing. W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
36. Schorey. J. S., Li, Q.. McCourt, D. W., Bong- Mastek, M., Clark-Curtiss, J. E., Ratliff, T. L. and Brown, E. J. A Mycobacterium leprae gene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect. Immun. 63(1995)2652-2657.
37. Sheehan, D. C. and Hrapchak, M. S. M. Carbohydrates. In: Theory and Practice of Histotechnology . 2nd ed. Sheehan, D. C., and Hrapchak, M. S. M., cds. St. Louis: The C. U. Mosby Company, 1980, pp. 159-179.
38. Shepard, C. J. R. and Shotton, D. M. Confocal Laser Scanning Microscopy . New York: Bios Scientific Publishers and Springer Verlag, 1997, pp. 1-10.
39. Takahashi, S., Dunn, M. A. and Seifter, S. Liver collagenase in murine schistosomiasis. Gastroenterology 78(1980)1425-1431.
40. Woeman, M. and Kasten, P. Polarized light microscopy in the study of the molecular structures of collagen and reticulin. Histochemistry 85(1986)41-49.
1. M.D., Ph.D., Laboratory of Leprosy, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Av. Brasil 4365, Manguinhos 21045-900. Rio de Janeiro. RJ, Brazil and Iguacu University, Nova Iguacu, Brazil.
2. M D., Ph.D.; Laboratory of Leprosy, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Av. Brasil 4365, Manguinhos 21045-900. Rio de Janeiro. RJ, Brazil and Iguacu University, Nova Iguacu, Brazil.
3. M.D., Laboratory of Leprosy, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, RJ. Brazil.
4. M.Sc.; Laboratory of Leprosy, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, RJ. Brazil.
5. M D.; Laboratory of Leprosy, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, RJ. Brazil.
6. M.D., Ph.D. Department of Pathology, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, RJ, Brazil.
Reprint request to Dr. Antunes at the Oswaldo Cruz Institute address above or FAX 55-21-270-9997; e-mail: santunes@gene.dbbm.fiocruz.br
Received for publication on 17 June 1997.
Accepted for publication in revised form on 6 January 1999.