- Volume 67 , Number 1
- Page: 35–45
Roles of tumor necrosis Factor-α and transforming growth Factor-β in regulating intercellular adhesion Molecule-1 expression on murine peritoneal macrophages Infected with M. leprae
ABSTRACT
Profiles of intercellular adhesion molecule-1 (ICAM-1) expression on murine peritoneal macrophages (MΦs) infected with Mycobacterium leprae during cultivation were examined with special reference to the regulatory effects of tumor necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β). When MΦs were infected with M. leprae or stimulated with heat-killed M. leprae at day 0, their ICAM-1 expression, measured in terms of the ratio of MΦs positively stained with anti-ICAM-1 antibody (Ab), rapidly increased, peaking during days 1 to 3 and thereafter fell, returning to the normal level by day 7. The addition of TNF-α or anti- TGF-β Ab inhibited the middle phase (day 7) downregulation of MΦ ICAM-1 expression, although the late-phase (day 14) downregulation of ICAM-1 was not prevented by them. M. leprae -infected MΦs released small amounts of TNF-α and significant amounts of TGF-β into the culture medium. This may indicate that M. leprae - infected MΦs produced the majority of TNF-α in a membrane-bound form. Alternatively, endogenous TNF-α might upregulate MΦ ICAM-1 expression even at very low concentrations. In any case, these findings indicate the central roles of TNF-α and TGF-β in the early phase upregulation and the middle-to-late phase downregulation, respectively, of ICAM-1 expression by M. leprae -infected MΦs.RÉSUMÉ
On a examiné le mode d'expression des molécules d'adhésion intercellulaire de type 1 (ICAM-1) chez dez macrophages (MΦs) infectés in vitro par Mycobacterium leprae , en tenant compte en particulier de la regulation d'expression exercée par le tumor necrosis factor-alpha (TNF-α) et le transforming growth factor-beta (TGF-β). Lorsque des M'Φs étaient infectés par M. leprae ou bien stimulés par des M. leprae tuées par la chaleur, l'expression de ICAM-1, mesurée en terme de proportion de MΦs marqués par des anticorps (Ac) anti- 1CAM-1, augmentait rapidement, présentait un pic entre le premier et le troisième jour après l'infection et diminuait ensuite, pour rejoindre le niveau basai d'expression vers le septième jour. L'addition de TNF-α ou d'anticorps anti-TGF-β inhibait la phase intermédiaire (septième jour) de retour au niveau basai d'expression d'ICAM-1 par les MΦs. Cependant, le retour tardif (quatorzième jour) au niveau de base d'expression de 1CAM-1 n'a pu être prévenu par ces molécules. Les MΦs infectés par M. leprae ont relargués dans le milieu de culture une petite quantité de TNF-α et une quantité plus importante de TGF-β. Cela pourrait suggérer que soit les MΦs infectés par M. leprae produiraient la majorité de leur TNF-α dans une forme assossiée aux membranes, soit que le TNF-α endogène pourrait stimuler l'expression de ICAM-1 même à très petite concentration chez les macrophages. En tout état de cause, ces trouvailles suggèrent un rôle central de TNF-α et de TGF-β, dans la stimulation précoce et dans le retour au niveau basai en phase moyenne et tardive, respectivement, de l'expression de ICAM-1 par les MΦs infectés par M. leprae.RESUMEN
Se examinó el efecto regulatorio del factor de necrosis tumoral alfa (TNFα) y del factor de crecimiento transformante beta (TGFβ) sobre la expresión de la molécula de adhesion intercelular ICAM-1 en macrófagos peritoneales murinos infectados con Mycobacterium leprae . Los macrófagos infectados con M. leprae o estimulados cons M. leprae muerto por calor en el dia 0, mostraron un rápido incrmeneto en la expresión de ICAM-1 que fue máxima entre los dias 1-3 y luego decayó, retomando a su nivel normal hacia el dia 7. La adición de TNFα o de un anticuerpo anti-TGF-β in- hibió la fase media (dia 7) de la expresión de ICAM-1 pero no modifico la fase tardia (dia 14). Los macrófagos infectados con M. leprae liberaron pequenas cantidades de TNFα y cantidades significativas de TGF-β en el medio de cultivo. Esto puede indicar que los macrófagos infectados por M. leprae produjeron la mayor parte del TNFα en una forma unida a membranas. Alternativamente, el TNFα endógeno podría regular la expresión de ICAM-1 aun a muy bajas concentraciones. En cualquier caso, estos hallazgos indican que el TNF-α y el TGF-β participan en la regulación de la expresión de ICAM en las fases temprana y tardia, respectivamente, de la infección por M. leprae.Adhesion molecules expressed on immunocompetent cells play important roles in cellular interactions for the development of immunological responses (24). Intercellular adhesion molecule-1 (ICAM-1) plays a role in lymphocyte trafficking by allowing ICAM-1-bearing endothelial cells to interact with leukocyte function-associated antigen-1 (LFA-1) on lymphocytes, and thereby facilitate the migration of lymphocytes to sites of inflammation along endothelial cells (24). Moreover, the interaction of LFA- 1 with ICAM-1 is critical for effective cellular interactions of T cells with antigen- presenting cells, including macrophages (MΦs), and the consequent activation of resting T cells (12, 15, 24). Indeed. ICAM-1 augments T-cell proliferation and is pivotal in recall antigen responses of T cells to purified protein derivative of Mycobacterium tuberculosis (30). Moreover, a recent study of Lopez Ramirez, et al . (14) showed that the ICAM-1 expression on THP-1 human monocyte-derived cell line was potently increased by stimulation with M. tuberculosis. In addition, the in vivo study by Sullivan, et al. (25) concerning M. leprae infection revealed that granulomas from both tuberculoid leprosy and lepromatous leprosy displayed ICAM-1 expression. This implies that ICAM-1 plays important roles in enhancing the immune and inflammatory responses to M. leprae . Therefore, it is of interest to know the profiles of ICAM-1 expression by MΦs after stimulation with M. leprae . In the present study, we found that ICAM-1 expression on murine peritoneal MΦs was increased during days 1 to 3 after stimulation with M. leprae because of autocrine upregulation by tumor necrosis factor-alpha (TNF-α). The ICAM-1 expression thereafter gradually decreased, returning to the normal level by day 7 due to the downregulating effect of transforming growth factor-beta (TGF-β) derived from M. leprae -stimulated MΦs themselves.
MATERIALS AND METHODS
Organisms. M. leprae were purified from the liver of an infected armadillo (#825) obtained from G. P. Walsh, Armed Forces Institute of Pathology, Washington, DC, U.S.A., by Percoll gradient centrifugation according to the method of Draper (IMMLEP protocol 1/79) with slight modifications (21). This M. leprae strain 825 contained 30% of living cells on the basis of fluorescein diacetate and ethidium bromide (FDA/EB) staining and 109 of the organisms contained 7.4 pmoles of ATP, indicating good viability of the bacterial preparation. In some experiments, M. leprae organisms were collected and purified from infected foot pads of B ALB/c nude mice, as previously reported (26). When 108 of these nude mouse M. leprae (strain Thai-53) were inoculated in the 7H12B medium of the BACTEC 460 Tuberculosis System and cultured according to Franzblau (11), the growth index (GI) value increased from 0 to 122 and 267 at day 4 and day 11, respectively, indicating good viability of this bacterial preparation. When BALB/c nude mice were infected with 106 of M. leprae Thai-53 into the hind foot pad, the organisms steadily grew at the sites of infection, reaching bacterial loads larger than 109 bacilli per foot pad within 1 year after infection (data not shown). This also indicates good viability of the bacterial preparation used.
Mice. Seven- to ten-week-old, male BALB/c mice purchased from Japan Clea Co., Osaka, Japan, were used.
Special agents for MΦ culture. Recombinant mouse TNF-α, ultrapure natural human TGβ-(3l and mouse anti-human TGβ-(3 monoclonal antibody (mAb) (also specific to mouse TGβ-(3) were purchased from Genzyme Co., Cambridge, Massachusetts, U.S.A. Rat anti-mouse TNF-α mAb was obtained from Pharmingen Co., San Diego, California, U.S.A. One unit of TNβ-a is defined as the amount required to mediate half-maximal cytotoxicity of L929 cells. All of these agents contained no preservative, such as sodium azide, and were found to be essentially free from LPS contamination by the Limulus J Single Test (Wako Pure Chemical Industry Co., Osaka, Japan).
Medium. Medium RPMI 1640 (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 25 raM HEPES, 2 mM glutamine, and 5% heat-inactivated fetal bovine serum (FBS) (M. A. Bioproducts, Walkersville, Maryland, U.S.A.) was used for cell culture unless otherwise specified. The medium and FBS used were found to be free from LPS contamination by the Limulus J Single Test.
Peritoneal MΦs. Peritoneal exudate cells (PECs) were collected with Hanks' balanced salt solution (HBSS) containing 2% FBS from mice given an intraperitoneal injection of 1 ml of peptone-starch 4 days before harvest. After washing with 2% FBS-HBSS by centrifugation at 200 x g x 5 min, the PECs were suspended in a small volume of the culture medium. Ten ml each of PEC suspension in 5% FBS-RPMI 1640 at the cell density of 5 x 106/ml was poured onto a 90-mm cell culture plate (Corning Glass Works Co., Corning, New York, U.S.A.) which was overlaid with 14-mm plastic sheets (about 20 sheets) (Wako Pure Chemical Industry Co., Osaka, Japan). After a 2-hr incubation at 37°C in a CO2 incubator (5% CO2-95% humidified air), the resultant sheets" were removed, thoroughly rinsed with 2% FBS-HBSS, and immersed into 1 ml of culture medium in a 16-mm cell culture well (24-wells; Corning). This method gave more than 90% pure MΦ cultures, with potent pinocytic ability for neutral red and phagocytic activity for latex beads.
Microscopic counting for ICAM-1- positive MΦs. MΦ monolayer cultures on the plastic sheets were cultured in 1.0 ml of 5% FBS-RPMI 1640 in 16-mm culture wells (Corning) in the presence or absence of 1 x 107/ml of M. leprae or latex beads (3.0-µm diameter; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) at 37°C in a CO, incubator for up to 14 days. At intervals, the MΦ monolayer cultures were removed, washed with phosphate buffered saline (PBS) containing 0.2% bovine serum albumin (BSA), fixed with 1% paraformaldehyde, incubated in 1% BSA-PBS at room temperature for 30 min, and then reacted with rat anti-mouse ICAM-1 mAb (Seika- gaku Corp., Tokyo, Japan) for 1 hr. After rinsing with 0.1% BSA-PBS, the MΦs were reacted with alkaline phosphatase-conjugated anti-rat Ig mAb (Seikagaku) for 1 hr and then washed with 0.1% BSA-PBS. Color development was achieved by using NBT-BCIP substrate (nitroblue tetra- zolium/5'-bromo-4-chloro-3-indoIylphos- phate) and the proportion of blue-stained MΦs (ICAM-1-positive cells) was enumerated by microscopic counting (16).
Cytokine measurement. The 1-, 3- or 7-day culture fluids of MΦs with or without M. leprae infection were measured for TNF-α and TGβ-(3 concentrations as previously described. Briefly, Immulon 4 plates (Dynatech Laboratories, Chantilly, Virginia, U.S.A.) were coated with a capture Ab for each cytokine using rat anti-mouse TNF-α mAb (Pharmingen) and mouse antihuman TGF-β mAb (also specific to mouse TGF-β) (Genzyme). Biotinylated rat anti- mouse TNF-α mAb (Pharmingen) and chicken anti-human TGF-β mAb (R&D System, Inc., Minneapolis, Minnesota, U.S.A.) were used as the detecting Abs. After binding of alkaline phosphatase-conjugated streptavidin (Life Technologies Co., Gaithersburg, Maryland, U.S.A.) in the case of TNF-α assay and alkaline phosphatase-conjugated rabbit anti-chicken/turkey IgGAb (Zymed Laboratories Inc., San Francisco, California, U.S.A.) in the case of TGF-β assay, color development wasachieved using p -nitrophenyl phosphate tablets (Sigma) as the substrate. Capture Ab and detecting Ab for each cytokine had different epitope specificities from each other.The present ELISA method was capable ofmeasuring the active form TGF-β in MΦ culture fluids in a specific fashion.
RESULTS
ICAM-1 expression on M. leprae -in-fected MΦs during cultivation. As shown in Figure 1, when MΦs were infected with M. leprae at day O (hatched bars), the ICAM-1 expression of cultured MΦs, in terms of increase in the ratio of ICAM-1-positive MΦs measured by the microscopic method, rapidly increased reaching the peak in the early phase (during day 1 to day3) of cultivation and thereafter decreased, returning to the normal levei by day 7. Alower increase in the ICAM-1 expression was also noted for uninfected (unstimulated) MΦs in the same period of time (open bars). In this experiment, residual numbers of attached MΦs on a plastic culture sheet during cultivation after M. leprae -infection (hatched bars) were changedas follows when the day O value was fixedas 100% (N = 5): day 1, 97.6 ± 5.5%; day 3,91.0 ± 7.1%; day 7, 78.9 ± 6.9%. This indicates that the reduction of ICAM-1 expression in the middle phase (day 7) of MΦ cultivation after M. leprae -infection was not due to M. leprae -mediated cytotoxicity. Figure 1 also shows that the addition of TGF-α at the concentration of 500 units/ml overcame the middle phase (day 7) down-regulation of the ICAM-1 expression (shaded bar), indicating that ICAM-1downregulation in the middle phase was due in pari to a defect of TGF-α. In this context, separate experiments showed that TGF-α, a crucial ICAM-1 upregulating cytokine (11, 20) displayed its ICAM- I upregu-lating effect on MΦs as follows.
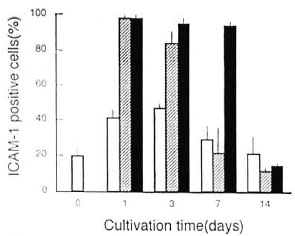
Fig. 1. Profiles of ICAM-I expression by M. leprae 825-infected MΦs during cultivation in medium with or without addition of exogenous TNF-α. = Uninfected MΦs were cultured in lhe absence of TNF-α; = M. leprae-infected MΦ were cultured in the absence of TNF-α were cultured in the presence of TNF-α added at 500units/1111 on day 0. Each bar indicates mean ± S.E.M.(N = 2).
First, as shown in Figure 2, a marked increase in the ICAM-1 expression was also observed for uninfected MΦs, when treated with exogenous TNF-α (500 units/ml), which was added on days 0, 2, and 6, in the early phase of cultivation (days 1 to 3) but not in the middle phase (day 7). Thus, in the case of uninfected MΦs, exogenous TNF-α could increase only their early phase ICAM-1 expression, differing from the case of M. leprae -'mitcitd MΦs (Fig. 1). Second, as shown in Figure 3, the addition of anti-TNF-α Ab (10 µg/ml) caused the reduction of the ICAM-1 expression by M. leprae -infected MΦs and this effect was most marked at day 3, confirming that MΦs- derived endogenous TNF-α plays the most important role in the early phase (around day 3) increase in the ICAM-1 expression by M. leprae -infected MΦs.
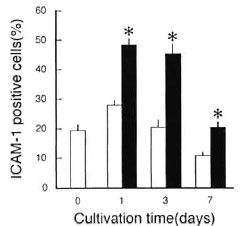
Fig. 2. Effects of exogenous TNF-α on ICAM-1 expression by uninfected MΦs during cultivation. MΦs were cultivated in the presence ( ) or absence (
) or absence ( ) of TNF-α which was added at 500 units/ml on days 0, 2, and 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 3). * Significantly larger than value of MΦs cultured in absence of TNF-α (p <0.05; Student's t test).
) of TNF-α which was added at 500 units/ml on days 0, 2, and 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 3). * Significantly larger than value of MΦs cultured in absence of TNF-α (p <0.05; Student's t test).
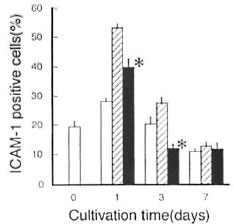
Fig. 3. Effects of anti-TNF-α Ab on ICAM-1 expression by M. leprae Thai-53-infected MΦs.  = Uninfected MΦs were cultured in the absence of anti- TNF-α Ab;
= Uninfected MΦs were cultured in the absence of anti- TNF-α Ab;  = M. leprae-infected MΦs were cultured in the absence of anti-TNF-oc Ab;
= M. leprae-infected MΦs were cultured in the absence of anti-TNF-oc Ab; = M. leprae-infected MΦs were cultured in the presence of anti-TNF-α Ab which was added at 10 µg/ml to the culture medium on days 0, 2. and 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 3). * Significantly smaller than the value of MΦs cultured in the absence of anti-TNF-α Ab (p <0.05; Student's t test).
= M. leprae-infected MΦs were cultured in the presence of anti-TNF-α Ab which was added at 10 µg/ml to the culture medium on days 0, 2. and 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 3). * Significantly smaller than the value of MΦs cultured in the absence of anti-TNF-α Ab (p <0.05; Student's t test).
The above findings indicate central roles of TNF-α in ICAM-1 upregulation of M. leprae -infected MΦs. However, as indicated in The Table only small amounts of TNF-α were released from M. leprae - infected MΦs into the culture medium during cultivation periods for up to 7 days. It thus appears that the majority of the TNF-α molecules were produced by M. leprae -infected MΦs in a membrane-bound form, molecular weight (MW) of 26 kD, but not in a released form (MW of 17 kD) (13). This membrane-bound form of TNF-α seems to play a crucial role in the ICAM-1 upregulation of M. leprae -infected MΦs.

Another important finding in Figure 1 is that TNF-α failed to overcome the late phase (day 14) downregulation of ICAM-1 expression. This phenomenon may be explained as follows. First, ICAM-1 reduction in the late phase seems to be due in part to nutrient deficiency in the MΦs culture medium, as previously demonstrated for M avium complex-infected MΦs (16). Second, it is plausible that, in the middle-to-late phase of cultivation, M.leprae -infected MΦs produced certain factors possessing an ICAM-1 downregulatory activity, as described below.
Role of TGF-β in the middle-to-late phase downregulation of ICAM-1 expression by M.leprae -infected MΦs. MΦs stimulated with mycobacterial organisms produce TGF-β, an immunosuppressive and MΦs-deactivating cytokine (4, 31), We previously found that the in vitro TGF-β production by murine peritoneal MΦs was initiated after day 3 and continued for up to at least day 14 when the MΦs were infected with M. avium complex (16). As indicated in The Table, significant levels of TGF-β were produced into the culture medium by MΦs regardless of M. leprae infection. However, it was also found that M. leprae-infected MΦs produced significantly larger amounts of TGF-β than did uninfected MΦs. Notably, anti-TNF-α Ab enhanced the TGF-β production by M. leprae -infected MΦs. In a separate experiment, the addition of 20 µg/ml of anti-TGF-β Ab caused an 82 ± 2% reduction of TGF-β accumulation into culture fluids of M. leprae - infected MΦs on day 3.
In addition to these findings, it has also been reported that TGF-β displayed down- regulatory effects on the interferon-gamma (IFN-γ)-induced ICAM-1 expression by rat microglia cells, which are cells of MΦ lineage (33). It is thus likely that TGF-β also plays some active role in ICAM-1 down- regulation observed in the case of M. leprae -infected MΦs. As shown in Figure 4, anti-TGF-β Ab significantly blocked the middle phase (day 7) downregulation of ICAM-1 expression by MΦs infected with M. leprae . In this case, anti-TGF-β Ab was added at 30 µg/ml on days 0, 3, and 6. In separate experiments, anti-TGF-β Ab at 30 µg/ml was found to neutralize 12 ng/ml of TGF-β, which is much larger than the amounts of TGF-β produced by M. leprae - infected MΦs (The Table).

Fig. 4. Effects of anti-TGF-β Ab on ICAM-1 expression by M. leprae 825-infected MΦs.  = Uninfected MΦs were cultured in the absence of anti-TGF-β Ab;
= Uninfected MΦs were cultured in the absence of anti-TGF-β Ab;  = M. leprae-infected MΦs were cultured in the absence of anti-TGF-β Ab;
= M. leprae-infected MΦs were cultured in the absence of anti-TGF-β Ab;  = M. leprae-infected MΦs were cultured in the presences of anti-TGF-β Ab which was added at 30 µg/ml to the culture medium on days 0, 3, and 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 3). * Significantly larger than value of MΦs cultured in absence of anti-TGF-β Ab (p <0.05; Student's t test).
= M. leprae-infected MΦs were cultured in the presences of anti-TGF-β Ab which was added at 30 µg/ml to the culture medium on days 0, 3, and 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 3). * Significantly larger than value of MΦs cultured in absence of anti-TGF-β Ab (p <0.05; Student's t test).
Figure 5 shows the effects of anti-TGF-β Ab on the ICAM-1 expression by MΦs stimulated with heat-killed (121°C, 15 min) M. leprae . In this case, anti-TGF-β Ab similarly blocked the middle phase (day 7) downregulation of ICAM-1, thereby indicating that profiles of ICAM-1 expression in MΦs stimulated due to infection with living M. leprae organisms were essentially the same as those in MΦs stimulated with heat-killed M. leprae .
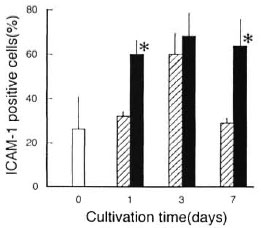
Fig. 5. Effects of anti-TGF-β Ab on ICAM-1 expression by MΦs stimulated with heat (121°C, 15 min)-killed M. leprae 825.  = ICAM-1 expression by normal MΦs before stimulation with heat-killed M. leprae;
= ICAM-1 expression by normal MΦs before stimulation with heat-killed M. leprae;  M. leprae-stimulated MΦs were cultured in the absence of anti-TGF-β Ab;
M. leprae-stimulated MΦs were cultured in the absence of anti-TGF-β Ab;  = M. leprae- stimulated MΦs were cultured in the presence of anti- TGF-β Ab which was added at 30 µg/ml to culture medium on days 0, 3, and 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 4). * Significantly larger than value of MΦs cultured in absence of anti- TGF-β Ab (p <0.05; Student's t test).
= M. leprae- stimulated MΦs were cultured in the presence of anti- TGF-β Ab which was added at 30 µg/ml to culture medium on days 0, 3, and 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 4). * Significantly larger than value of MΦs cultured in absence of anti- TGF-β Ab (p <0.05; Student's t test).
Effects of addition of TNF-α or anti- TGF-β Ab on ICAM-1 expression by latex beads-phagocytosing MΦs. In order to know whether or not TNF-α-mediated upregulation and TGF-β-mediated down- regulation of MΦ ICAM-1 expression observed in Figures 1, 4, and 5 was specifically caused by M. leprae infection, we examined the effects of the addition of TNF-α and anti-TGF-β Ab on the ICAM-1 expression by MΦs stimulated by phagocytosis of latex beads. For this purpose, MΦs were cultured with 3-µm latex beads which are known to be taken up by MΦs through phagocytosis. As shown in Figure 6, the ICAM-1 expression by latex beads phagocytizing MΦs did not reduce so markedly at day 7 as in the case of M.leprae -infected MΦs (Figs. 1, 3, and 4). Moreover, neither TNF-α nor anti-TGF-β Ab significantly affected the ICAM-1 expression by latex beads phagocytizing MΦs.
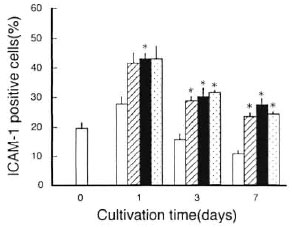
Fig. 6. Profiles of ICAM-1 expression by latex beads-phagocytizing MΦs during cultivation.  = Day 0: ICAM-1 expression by normal MΦs before latex phagocytosis. Days 1, 3, 7: ICAM-1 expression by control MΦs without phagocytizing latex particles after cultivation in medium without addition;
= Day 0: ICAM-1 expression by normal MΦs before latex phagocytosis. Days 1, 3, 7: ICAM-1 expression by control MΦs without phagocytizing latex particles after cultivation in medium without addition;  = latex beads-phagocytizing MΦs cultured in medium without addition;
= latex beads-phagocytizing MΦs cultured in medium without addition;  and
and  = latex beads-phagocytizing MΦs cultured in presence of either TNF-α (
= latex beads-phagocytizing MΦs cultured in presence of either TNF-α ( ) or anti-TGF-β Ab (
) or anti-TGF-β Ab (  ). TNF-α and anti-TGF-β Ab added to culture medium at 500 units/ml and 10 µg/ml, respectively, on days 0, 2, 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 3). * Significantly larger than the value of control MΦs (p ,0.05; Student's t test).
). TNF-α and anti-TGF-β Ab added to culture medium at 500 units/ml and 10 µg/ml, respectively, on days 0, 2, 6 during cultivation. Each bar indicates mean ± S.E.M. (N = 3). * Significantly larger than the value of control MΦs (p ,0.05; Student's t test).
DISCUSSION
The present study indicated that stimulation of murine peritoneal MΦ with M. leprae caused an upregulallon of ICAM-1 ex-pression by the MΦ in the early phase(days 1 to 3) of cultivation. However, theICAM-1 expression thereafter rapidly decreased, and returned to the normal leveiby day 7. This profile of change in MΦ ICAM-1 expression during the course ofcultivation seems to be peculiar to M. leprae -infected MΦs, since such marked de-crease in the ICAM-1 expression at day 7was not observed for MΦ stimulated dueto phagocytosis of latex beads (Fig. 6). Themiddle phase (day 7) downregulation of ICAM-1 expression appeared to be due inpari to a defect of M(I)-derived TNF-α having upregulating effects on M(I) ICAM-1 expression (14, 20), since supplementary addition of TNF-α prevented the middle (day7) phase reduction of ICAM-1 expression (Fig. 1).
This concept is supported by the following findings obtained in separate experimenta. First, exogenous TNF-α increased the ICAM-1 expression on uninfected MΦ (Fig. 2). Second, anti-TNF-α Ab abolished the M. leprae infection-induced increase in MΦ expression of TNF-α in the early phase (day 3) of cultivation (Fig. 3). However, under the present experimental condition, wedetected only low leveis of TNF-α releasedfrom M. leprae -infected M(Ds roto the culture medium (The Table). This may meanthe individual M. leprae -infected MΦs produced TNF-α mainly in a membrane-bound form with a MW of 26 kD but not in a releasing form with a MW of 17 kD (13, 27),and that such membrane-bound TNF-α molecules transfer their stimulatory signalsto other MΦ through cell-to-cell contact. Alternatively, it is also possible that MΦ-derived endogenous TNF-α could inducethe ICAM-1 upregulation of M. leprae -infected MΦs even at very low concentrations.
It appears that an immunoregulatory cytokine, TGF- β (3 (31) also plays an importantrole in the ICAM-1 downregulation of theMΦ, since anti-TGF- β (3 Ab overcame the middle phase (day 7) downregulation ofthe ICAM-1 expression by MΦ infectedor stimulated with M. leprae (Figs. 4 and 5). In this context, anti-TGF-β (3 Ab failed toincrease the TNF-α released by M. leprae -infected MΦs (The Table). Therefore,TGF-β-mediated downregulation of MΦ ICAM-1 expression is not attributable to the reduction of MΦ TNF-α-producing ability due to TGF-β action. Moreover, although a significam levei of the TGF-β production by M. leprae -infected MΦs was observed at day 3, ICAM-1 downregulationwas not observed at this time point (TheTable versus Figs. 1 and 4). This suggests that TGF-β could not effectively suppress the ICAM-1 expression by MΦ which were actively producing ICAM-1 upregulating factors, including TNF-α. Moreover, it seems that MΦ factors other than TGF-β also contribute to the middle-to-late phase downregulation of the ICAM-1 expression. For instance, the antiinflammatory cytokine interleukin 10 (IL-10) (8, 19, 22) and, also, prostaglandin E, may play some roles in the ICAM-1 downregulation. Indeed, our recent study on M. avium complex-infected MΦs revealed that the middle (day 7) to late (day 14) phase ICAM-1 expression by M. avium complex-infected MΦs is potently suppressed by these MΦ factors in an autocrine fashion (Tomioka, et al .: manuscript submitted). Thus, it is of interest to examine the participation of these and other unknown ICAM-1 downregulating factors in the late-phase reduction of MΦ ICAM-1 expression, with special reference to their collaborating effects.
It is somewhat enigmatic to note that both exogenous TNF-α and anti-TGF-β Abs fully overcame the middle phase (day 7) downregulation of ICAM-1 expression on M. leprae -infected MΦs (Figs. 1 and 4). Although the precise reason for this situation is unknown, it may be explained as follows. First, it is thought that by receiving high levels of activating signals of TNF-α MΦs might become resistant to ICAM-I expression downregulatory effects of TGF-β. If this was the case, the addition of exogenous TNF-α in excess amounts might cause the effective recovery of MΦ ICAM-1 expression in the middle phase (day 7) by protecting M. leprae -infected MΦs from TGF-β-mediated ICAM-1 downregulating effects despite the fact that significantly high amounts of TGF-β were produced and released by the MΦs into the culture medium at the same time point. Second, it is also thought that TNF-α-mediated activating signals suppress TGF-β producing ability of M.leprae -infected MΦs, since anti-TNF-α Ab was found to decrease MΦ TGF-β production (The Table). Therefore, TNF-α treatment of M. leprae -infected MΦs might antagonize TGF-β-mediated ICAM-1 downregulation by reducing the production of MΦ TGF-β, causing a recovery of MΦ ICAM-1 expression.
Notably, the profiles of ICAM-1 expression in MΦs stimulated with living M. leprae organisms were essentially the same as those in MΦs stimulated with heat-killed bacilli (Figs. 4 and 5). This indicates that heat-stable components of M. leprae play roles not only in the early-phase upregulation ICAM-1 expression but also in the middle-to-late phase downregulation of ICAM-1 expression. First, lipoarabino- mannan (LAM) is one of the major candidates for the ICAM-1-modulating components of M. leprae , since mycobacterial LAM is capable of upregulating MΦ ICAM-1 expression (14). It has been reported that arabinosylated LAM of M. tuberculosis induces MΦ production of proinflammatory cytokines, including TNF-α and IL-1 (2, 5, 7, l8), which are the major mediators of ICAM-1 upregulation (10, l4, 20). Nevertheless, in the present study, M. leprae -infected MΦs did not produce detectable amounts of TNF-α in the culture medium (The Table). In this context, Adams, et al . (1) reported that LAM from M. leprae having mannose capping did not induce TNF-α production by MΦs, as in the case of mannosylated LAM from virulent M. tuberculosis Erdman strain (LAM Erdman) (6). However, they also found that mannosylated LAM, Erdman could trigger MΦ antimicrobial activity and elevated levels of MΦ nitric oxide production, and that this triggering capacity of LAM Erdman was abrogated by an anti-TNF-α Ab, indicating that TNF-α is involved in the induction of MΦ activation by LAMErdman . Notably, LAMErdman did so without inducing elevated levels ol TNF-α in the supernatant medium. As discussed above, it thus appears that the LAM of M. leprae triggered MΦs to produce TNF-α in a membrane- bound form (MW = 26 kD) (13), and such membrane-bound TNF-α molecules transferred their stimulatory signals from one MΦ to another MΦ through cell-to-cell contact. Indeed, it has been demonstrated that activated MΦs could kill a TNF-α- sensitive tumor cell line in the absence of any demonstrable TNF-α in the culture supernatants and this killing was attributed to the presence of cell surface-associated TNF-α (9). In addition, segments of M. leprae cell-wall skeleton, such as mycolyl-arabinogalactan-peptidoglycan complex, protein-peptidoglycan complex, and muramyl dipeptide, may play roles in the upregulation of ICAM-1 expression by M. leprae - infected MΦs since these substances are known to trigger elevated production of TNF-α (3).
Second, mycobacterial LAM also induces MΦ production of immunoregulatory cytokines, including TGF-β and IL-10 (7, 29) which have ICAM-1 downregulating functions (23, 32) suggesting the roles of LAM in the ICAM-1 downregulation of M. leprae - infected MΦs in the middle-to-late phase of cultivation. In addition, it is also likely that other cellular components, such as heat-stable proteins, play roles in the ICAM-1 modulation since purified protein derivative (PPD) of M. tuberculosis is reported to up- regulate MΦ production of TNF-α, IL-1, and, moreover, TGF-β (10, 29).
Moncada, et al . (I7) reported that the epidermis of leprosy patients with inflammatory skin diseases exhibited an increased ICAM-1 expression. They also found a lack of ICAM-1 expression in the epidermis, especially karatinocytes (cells with MΦ-like functions), of lepromatous patients. It is of interest to know whether or not the defective expression of ICAM-1 in the lesional epidermis of lepromatous leprosy patients is mediated by TGF-β. In this context, we previously found sequential increase of tissue levels of TNF-α, IL-10 and TGF-β in spleens during the course of M. avium complex infection in mice (28). That is, the increase in the TNF-α level was observed around week 2 after infection, followed by an elevated level of IL-10 during weeks 2 to 4, and the increase of TGF-β level was subsequently observed after week 4 for at least up to week 8. Further studies are currently underway to examine the roles of ICAM-1 downregulating cytokines, including TGF-β and IL-10 as mediators of defective ICAM- 1 expression in skin lesions of lepromatous leprosy patients.
Acknowledgment. This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan, and by the United States-Japan Cooperative, Medical Science Program (Tuberculosis and Leprosy Section).
REFERENCES
1. Adams, L. B., Fukutomi, Y. and Krahenbuhl, J. L. Regulation of murine macrophage effector functions by lipoarabinomannan from mycobacterial strains with different degrees of virulence. Infect. Immun. 61 (1993)4173-4181.
2. Barnes, P. F., Chatterjee, D.. Aisrams, J. S., Lu, S., Wang, E., Yamamura, M., Brennan, P. J. and Modlin, R. L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan; relationship to chemical structure. J. Immunol. 149(1992)541-547.
3. Barnes, P. F., Chatterjee, D., Brennan, P. J., Rea, T. H. and Modlin, R. L. Tumor necrosis factor production in patients with leprosy. Infect. Immun. 60(1992) 1441-1446.
4. Bermudez, L. E. Production of transforming growth factor-P by Mycobacterium avium -infected human macrophages is associated with unresponsiveness to IFN-γ. J. Immunol. 150 (1993) 1838-1845.
5. Bradbury, M. G. and Moreno, C. Effect of lipoarabinomannan and mycobacteria on tumor necrosis factor production by different populations of murine macrophages. Clin. Exp. Immunol. 94 (1993)57-63.
6. Chatterjee, D., Roberts, A. D., Lowell, K., Brennan, P. J. and Orme, I. M. Structural basis of capacity of lipoarabinomannan lo induce secretion of tumor necrosis factor. Infect. Immun. 60 1249-1253.
7. Dahl, K. E., Shiratsuchi, H., Hamilton, B. D., Ellnkr, J. J and Toosi, Z. Selective induction of transforming growth factor β in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis . Infect. Immun. 64 (1996)399-405.
8. De Waal Malf.fyt, R., Yssel, H., Roncarolo, M.-G., Spits. H. and Vries, J. E. Interleukin-10. Curr. Opin. Immunol. 4 (1992)314-320.
9. Decker, T., Lohmann-Matthes, M.-L. and Giford, G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J. Immunol. 138 (1987) 957-962.
10. Dustin, M. L., Rothlein, R., Bhan, A. K., Dinarello, C. A. and Springer, T. A. Induction by IL 1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J. Immunol. 137 (1986)245-254.
11. Franzblau, S. G. Drug susceptibility testing of Mycobacterium leprae in the BACTEC 460 System. Antimicrob. Agents Chemother. 33 (1989) 2115-2117.
12. Hagerty, D. T. Intercellular adhesion molecule-1 is necessary but not sufficient to activate CD4* T cells. J. Immunol. 156 (1996)3652-3659.
13. Kriegler, M., Perez, C., DeFay, K., Albert, I. and Lu, S. D. A novel form of TNF/cachectin is a surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell 53 (1988)45-53.
14. Lopez Ramirez, G. M., Rom, W. N., Ciotoli, C., Talbot, A., Martiniuk, F., Cronstf.n, B. and Reibman, J. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect. Immun. 62 (1994) 2515-2520.
15. Marlin, S. D. and Springer, T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen-1 (LFA-1). Cell 51 (1987)813-819.
16. Maw, W. W., Tomioka, H., Sato, K. and Saito, H. The expression of ICAM-1 on macrophages stimulated with Mycobacterium avium complex and its control by some regulatory cytokines. Kekkaku 71 (1996)561-567.
17. Moncada, B., Torres-Alvarez, M. B., Gonzalez- Amaro, R., Huentes-Ahumada, C., Baranda, L., Delgado, S. P. and Garcia, R. Lack of expression of intercellular adhesion molecules ICAM-1 in lepromatous leprosy patients. Int. J. Lepr. 61 581-585.
18. Moreno, C., Taverne, J., Mehlert, A., Bate, C. A.. Brealey, R. J., Meager, A.. Rook, G. A. and Playkair, J. H. Lipoarabinomannan from Mycobacterium tuberculosis induced the production of tumor necrosis factor from human and murine macrophages. Clin. Exp. Immunol. 76 (1986)240-245."
19. Murray, P. J., Wang, L., Onueryk, C., Tepper, R. I. and Young, R. A. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J. Immunol. 158 (1997)315-321.
20. Rothlein, R., Czajkowski, M., O'Nell, M. M., Mari.in, S. D., Mainolr, E. and Merluzzi, V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. J. Immunol. 141 (1988)1665-1669.
21. Saito, H., Tomioka, H. and Sato, K. Purification of M. leprae with special reference to the effects of purified M. leprae vaccines on host macrophage cell functions. Jpn. J. Lepr. 56 (1987)101-109.
22. Seyler, I., Appel, M., Devissagguet, J.-P, Legrand, P. and Barratt, G. Modulation of nitric oxide production in RAW 264.7 cells by transforming growth factor-beta and interleukin-10: differential effects on free and encapsulated immunomodulator. J. Leukoc. Biol. 62 (1997)374-380.
23. Siirikant, P., Weber, E., Jilling, T. and Ben- veniste, E. N. Intercellular adhesion molecule-1 gene expression by glial cells. Differential mechanisms of inhibition by IL-10 and IL-6. J. Immunol. 155 (1995)1489-1501.
24. Springer, T. A. Adhesion receptors of the immune system. Nature 346 (1990)425-434.
25. Sullivan, L., Sano, S., Pirmez, C., Salgame, P., Mueller, C., Hoeman, F., Uyemura, K., Rea, T. H.. Bloom, B. R. and Modlin, R. L. Expression of adhesion molecules in leprosy lesions. Infect. Immun. 59 (1991)4154-4160.
26. Tomioka, H., Saito, H. and Sato, K. Evaluation of BACTEC 460 TB System for measurement of in vitro axAi- Mycobacterium leprae activity of various antimicrobials. Jpn. J. Lepr. 61 (1992)157-164.
27. Tomioka, H., Sato, K., Maw, W. W. and Saito, H. The role of tumor necrosis factor, interferon-y, transforming growth factor-β, and nitric oxide in the expression of immunosuppressive functions of splenic macrophages induced by Mycobacterium avium complex infection. J. Leukoc. Biol. 58 (1995)704-712.
28. Tomioka, H., Sato, K., Shimizu, T. Sano, C., Akaki, T., Saito, H., Fujii, K. and Hidaka, T. Effects of benzoxazinorifamycin KRM-1648 on cytokine production at sites of Mycobacterium avium complex infection induced in mice. Antimicrob. Agents Chemother. 41 (1997)357-362.
29. Toossi, Z., Young, T. G., Averill, L. E., Hamilton, B. D., Shiratsuchi, H. and Ellner, J. J. Induction of transforming growth factor β1 by purified protein derivative of Mycobacterium tuberculosis . Infect. Immun. 63 (1995)224-228.
30. Van Seventer, G. A., Shimizu, Y, Horgan, K. J. and Shaw, S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J. Immunol. 144 (1990)4579-4586.
31. Waul, S. M. Transforming growth factor beta (TGF-β) in inflammation: a cause and a cure. J. Clin. Immunol. 12 (1992)61-73.
32. Willems, F., Marchant, A., Delville, J.-P., Gerard, C., Delaux, A., Velu, T., de Bore, M. and Goldman, M. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur. J. Immunol. 24 (1994)1007-1009.
33. Xiao, B.-G., Zhang, G.-X., Ma, C.-G. and Link, H. Transforming growth factor-beta 1 (TGF-β) - mediated inhibition of glial cell proliferation and downregulation of intercellular adhesion molecule-1 (ICAM-1) are interrupted by interferon- gamma (IFN-γ). Clin. Exp. Immunol. 103 (1996)475-481.
1. M.S.; Department of Microbiology and Immunology, Shimane Medical University, Izumo, Shimane 693, Japan.
2. M.D., Ph.D.; Department of Microbiology and Immunology, Shimane Medical University, Izumo, Shimane 693, Japan.
3. Ph.D.. Department of Microbiology and Immunology, Shimane Medical University, Izumo, Shimane 693, Japan.
Reprint requests to Dr. H. Tomioka at the above address or FAX 81-853-20-2145.
Received for publication on 25 November 1997.
Accepted for publication in revised form on 10 December 1998.