- Volume 67 , Number 2
- Page: 119–28
Patient contact is the major determinant in incident leprosy: implications for future control
ABSTRACT
Notwithstanding the elimination efforts, leprosy control programs face the problem of many leprosy patients remaining undetected. Leprosy control focuses on early diagnosis through screening of household contacts, although this high-risk group generates only a small proportion of all incident cases. For the remaining incident cases, leprosy control programs have to rely on self-reporting of patients. We explored the extent to which other contact groups contribute to incident leprosy. We examined retrospectively incident leprosy over 25 years in a high-endemic village of 2283 inhabitants in Sulawesi, Indonesia, by systematically reviewing data obtained f rom the local program and actively gathering data through interviews and a house-to-house survey. We investigated the contact status in the past of every incident case. In addition to household contact, we distinguished neighbor and social contacts. Of the 101 incident cases over a 25-year period, 79 (78%) could be associated to contact with another leprosy patient. Twenty-eight (28%) of these 101 cases were identified as household contacts, 36 (36%) as neighbors, and the remaining 15 (15%) as social contacts. Three patients had not had a traceable previous contact with another leprosy patient, and no information could be gathered f rom 19 patients. The median span of time f rom the registration of the primary case to that of the secondary case was 3 years; 95% of the secondary cases were detected within 6 years after the primary case. The estimated risk for leprosy was about nine times higher in households of patients and four times higher in direct neighboring houses of patients compared to households that had had no such contact with patients. The highest risk of leprosy was associated with households of multibacillary patients. The risk of leprosy for households of paucibacillary patients was similar to the risk of leprosy for direct neighboring houses of multibacillary patients, indicating that both the type of leprosy of the primary case and the distance to the primary case are important contributing factors for the risk of leprosy. Contact with a leprosy patient is the major determinant in incident leprosy; the type of contact is not limited to household relationships but also includes neighbor and social relationships. This finding can be translated into a valuable and sustainable tool for leprosy control programs and elimination campaigns by focusing case detection and health promotion activities not only on household contacts but also on at least the neighbors of leprosy cases.RÉSUMÉ
En dépit des efforts d'éradication. les programmes de contrôle contre la lèpre sont confrontés au problème de la non-détection de nombreux patients lépreux. Le contrôle de la lèpre se focalise sur le diagnostic précoce des personnes contactes habitant sous le même toit, bien que ce groupe à haut risque ne génère qu'une faible proportion des nouveaux cas détectés. Pour le reste, les programmes de contrôle contre la lèpre doivent compter sur l'initiative des patients euxmêmes. Nous avons voulu explorer l'importance des autres groupes contacts dans la perspective de leur contribution à l'incidence de la lèpre. Nous avons rétrospectivement examiné l'incidence de la lèpre sur une période de 25 années dans un village hautement endémique de 2283 habitants dans le Sulawesi, en Indonésie. Nous avons fait une revue systématique des données obtenues à partir du progamme local et nous avons activement rassemblé des données en menant des interviews et des enquêtes maison par maison. Nous avons cherché à définir le plus précisément possible le statut de personne contacte de chaque nouveau cas rapporté. En plus des personnes contactes habitant sous le même toit, nous avons distingué les voisins et les contacts sociaux. Parmi 101 nouveaux cas diagnostiqués sur une période de 25 ans, 79 (78%) ont pu être reliés à un contact avec un autre patient lépreux. Vingt huit (28%) de ces 101 cas furent associés à des personnes contactes habitant sous le même toit, 36 (36%) à des voisins et 15 (15%) restant à des contacts sociaux. Trois patients n'ont pas eu de contact documenté antérieur au diagnostic avec un autre patient lépreux, et aucune information n'a pu être rassemblée pour 19 patients. La médiane statistique du laps de temps entre les enregistrements du premier cas et du deuxième cas était de 3 années; 95% des deuxièmes cas furent détectés dans les 6 années suivant le premier cas. Le risque de développer la lèpre fut estimé ête environ 9 fois plus élevé pour les compagnons de foyers d'habitation vivant sous le même toit que des patients hanséniens et 4 fois plus élevé pour les maisons voisines directes des patients, comparé aux foyers d'habitations n'ayant pas de tels contacts avec des patients. Le risque le plus élevé de développer la lèpre fut associé à un foyer d'habitation ayant un patient multibacillaire. Le risque de contatcr la lèpre parmi les membres d'un foyer hébergeant un patient paucibacillaire était similaire au risque pour les maisons voisines d'un patient multibacillaire, indiquant qu'à la fois le type de lèpre et la distance du premier cas sont des facteurs contribuant au risque de développer la lèpre. Le contact avec un patient lépreux est le facteur déterminant pour l'incidence de la lèpre; le type de contact n'est pas limité aux seuls membres d'un foyer d'habitation mais inclue les voisins et les relatifs issus d'activités sociales. Ces données peuvent être utilement prises en compte par les programmes de contrôle contre la lèpre et les campagnes d'éradication pour concentrer leurs efforts de détection et de promotion de la santé, non seulement sur les personnes contactes vivant sous le même toit, mais aussi au moins sur les voisins des cas de lèpre.RESUMEN
No obstante los esfuerzos de erradicación, los programas de control de la lepra se enfrentan al problema de que muchos pacientes permanecen sin ser detectados. El control de la lepra se enfoca al diagnóstico temprano a través del examen de contactos convivientes, aunque este grupo de alto riesgo genera sólo una pequenã porción de todos los casos incidentes. Para el resto de los casos incidentes, los programas de control de la lepra deben de confiar en los autorreportes de los pacientes. Aquí. nosotros exploramos el grado en el que otros grupos de contactos contribuyen a la incidência de la lepra. Estudiamos retrospectivamente la incidência de la lepra en los pasados 25 años en una población de alta endemia de 2283 habitantes, en Sulawesi, Indonesia. Para colectar los datos, se revisaron sistemáticamente los archivos dei programa local, se entrevistaron personas y se hicieron exploraciones de casa en casa. Se investigó la condición de contacto en el pasado de cada caso incidente. Además de los contactos convivientes también tomamos en cucnta a los contactos vecinos y a los contactos sociales. De los 101 casos incidentes en un periodo de 25 anos, 79 (78%) pudieron reconocerse como contactos de otro caso de lepra. Veintiocho (28%) de estos 101 casos se identificaron como contactos convivientes, 36 (36%) como vecinos, y los 15 restantes (15%) como contactos sociales. Tres de los pacientes no habian tenido contacto con otro enfermo de lepra y no se pudo obtener información de 19 pacientes. El lapso de tiempo promedio entre el registro dei caso primário y el dei caso secundário fue de 3 años; 95% de los casos secundários fueron detectados en los 6 años siguientes al registro dei caso primário. El riesgo estimado fue aproximadamente 9 veces mayor en los convivientes de los pacientes y cuatro veces más alto entre los vecinos directos, que en los indivíduos que no habian tenido contacto con los pacientes. El riesgo más alto de adquirir la enfermedad estuvo asociado con los convivientes de los pacientes multibacilares. El riesgo de lepra para los convivientes de pacientes paucibacilares fue similar al riesgo de los vecinos directos de casos multibacilares, indicando que tanto el tipo de lepra corao la distancia del caso primario son factores importantes que contribuyen al riesgo de la enfermedad. Un contacto de un paciente con lepra es el principal déterminante de la lepra incidente; el lipo de contacto no esta limitado al entorno intrafamiliar, también contribuyen las relaciones vecinales y sociales. Este hallazgo puede ser de utilidad en cl diseño de los programas de contrat y de erradieación de la lepra ya que dériva de un estudio enfocado a la deteceión de casos y a la promoción de la salud, no sólo entre los contactos convivientes sino también entre los convivicntes vecinales (y sociales) de los casos de lepra.The introduction of the relatively short multidrug therapy (MDT) regimens for leprosy, an infection caused by Mycobacterium leprae , has resulted in a sharp decrease in the number of registered leprosy patients in the world (7). Encouraged by this success, the World Health Organization (WHO) has adopted the goal of the elimination of leprosy as a public health problem by the year 2000, defined by a world-wide prevalence of below l/l0,000. Leprosy seems thereby to have been reduced to a tangible health problem.
The major hindrance in leprosy control and thereby in reaching the elimination goal is that many leprosy cases remain undetected for a long period (7)Many of these patients are a continuous source of infection and thus keep transmission ongoing. Mass surveys to detect actively all new patients are not cost-effective and are, therefore, not routinely used by leprosy control programs. Many programs have restricted active case finding among household contacts of newly detected leprosy patients, since this group has an enhanced risk of disease, as was shown in the early 1940s (2,5)
A recent study showed that individuals living in the same household as a multibacillary leprosy patient have a fiveto eightfold higher risk of developing the disease compared with individuals without household contact (3). However, household contacts generate only a small proportion of all incident cases; in the study of Fine, et al . (3) only 15%-30% of the incident cases could be attributed to household contact. This poses an incongruity that the majority of new cases of this infectious disease seem to arise from the noncontact group.
Leprosy control programs restricting themselves to active case finding among household contacts thus consistently overlook the majority of incident cases. How can this incongruity be solved and how can new leprosy cases in a community be detected more effectively?
To answer these questions we examined retrospectively over 25 years incident leprosy in a high-endemic village in Indonesia by systematically reviewing data obtained from the local program and by actively gathering data through interviews and a house-to-house survey. We broadened the definition of contact from household contact to neighbor, family and social contact, and we explored the extent to which these other contact groups contribute to incident leprosy.
MATERIALS AND METHODS
The study took place in Tumaluntung, a village of 2283 inhabitants (1996 census) situated in the Province of North Sulawesi in Indonesia. The village is situated in the subdistrict Kauditan which is known as a high leprosy endemic area with an average case-detection rate of more than 5 per 10,000 in the previous years. The population has a fairly homogeneous socioeconomic status and the majority are subsistence farmers. In this village we studied the relationships between the registered incident leprosy cases over a 25-year period from 1971-1996. Information on these patients was obtained through analysis of the patient cards kept on registration by the local leprosy control program, by interviews with inhabitants and local leprosy workers, and through a house-to-house survey conducted in 1996.
The leprosy registration started here in 1971. Prior to the implementation of MDT, which replaced dapsone monotherapy, the leprosy control program first carried out a mass survey in 1982 to detect new cases of leprosy. Because of the high incidence of leprosy in this village the program adopted the policy of active case finding through routine contact surveys, chase surveys and mass surveys (conducted in 1988 and in 1991). Apart from these actively detected patients, the register contained self-reported leprosy cases.
We recorded the age, sex, clinical classification and date of registration for all registered leprosy patients since 1971. Through house visits and interviews the place of residence at the time of and prior to the time of registration was determined for every incident case. This information was verified by relating the dates of registration and the places of residence by life events such as births, marriages and deaths, and by cross checking with the information obtained from patient cards and from interviews with third parties (leprosy workers, village health workers, village community leaders and relatives). In addition, information on the kind and span of any relationships with other leprosy patients was obtained from incident cases and was cross checked. If a patient had died or moved out of the village, all the above information was obtained from a close relative. Nonconfirmed data were not used in the analysis. Leprosy patients were diagnosed and classified as multibacillary (MB) or paucibacillary (PB) according to provincial guidelines within the framework of the operational control program, which was usually on clinical criteria only. Bacteriological examination of slit-skin smears was carried out for only a minority of patients.
We categorized the contact status of incident leprosy patients hierarchically into household, neighbor, and social contacts. We defined incident cases as household contacts when they had lived with another patient in the same household at the time of registration of this primary case. Incident leprosy cases were defined as neighbors when they had lived next to the house of another patient at the time of registration of this primary case. Neighbors 1 were those living in one of the directly adjacent houses (usually: one to the left, one to the right and one directly across the street); neighbors 2 were those living directly next to the neighbor 1 houses.
Apart from household and neighbor contacts, we classified incident cases as relative, social (close friends) or business contacts. Relatives were defined as first, second and third-degree family members of a leprosy patient; close friends were those who reported to having a special relationship in terms of daily social contact (like visiting and eating together) with a leprosy patient; business contacts were those who, because of a professional linkage (working in the same place), had daily contact with a leprosy patient at the time when the primary case was registered. Eligibility criteria into any of the above-mentioned categories of contact also included the requirement that the duration of contact lasted at least 3 months.
In order to make an estimation of the risk for developing leprosy for the different categories of contact, we determined first when the house and neighboring houses of a new case of leprosy were "at risk" for having another new case of leprosy in the household. We took 6 years as a period of risk since most of the new cases were detected within a 6-year period (see Results section). We categorized hierarchically the houses of household contacts and of neighbor contacts at risk and counted the cumulative years at risk for these categories. Subsequently, the number of cases in this period for each category was divided by the total number of risk years and expressed in new cases per 100 household-years at risk.
RESULTS
During the period 1971 to 1996 a total of 110 leprosy patients was detected in the register of the leprosy control program. Nine of them were registered twice; 5 were having a relapse and 4 of them had not completed their treatment after the first registration, leaving 101 incident cases. In the house-to-house survey performed in 1996, 1450 people (63%) were examined for clinical signs of leprosy; six new patients were found. This increased the total to 107 patients. We found an almost equal distribution of females (50.5%) and males (49.5%), and PB (52%) and MB (48%) patients. There was no difference in the age distribution between males and females; the mean age for all patients was 46.4 years (median 52 years).
The residence of all 107 cases at the time of their registration was traced. Six patients were excluded from the study because they had been diagnosed with leprosy before they moved into the village of Tumaluntung. The residences of seven patients could not be traced with certainty. Figure 1 shows the mapping of the remaining 94 incident cases in their houses. They had been living in a total of 68 houses, so that 13% of all the houses (N = 522) in the village were, over the 25 years, ever occupied by leprosy patients. The number of patients per house varied from 1 to 5 and the distribution of patients over the houses showed a highly significant clustering (chi-squared test for heterogeneity p <10-4).
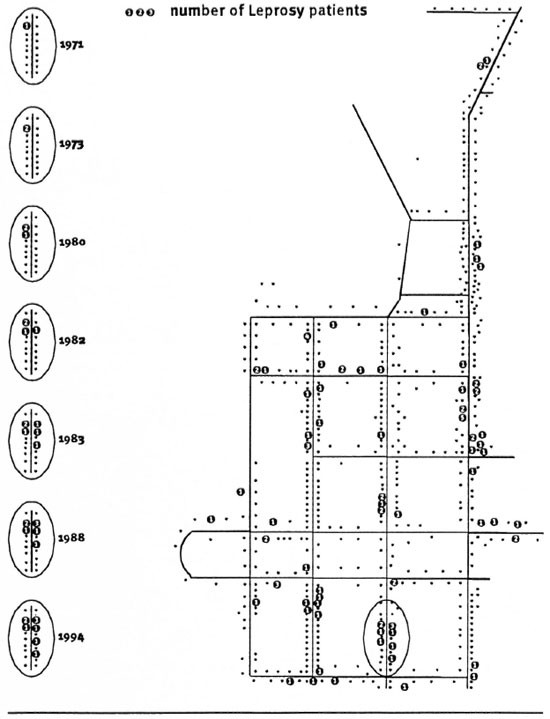
Fig. 1. Schematic of Tumaluntung village. Residentes where one or more Ieprosy patients resided during the25-year study are indicated. Left hand lide of the figure shows, as an example, the occurrence of incident patientsin time of one cluster of houses.
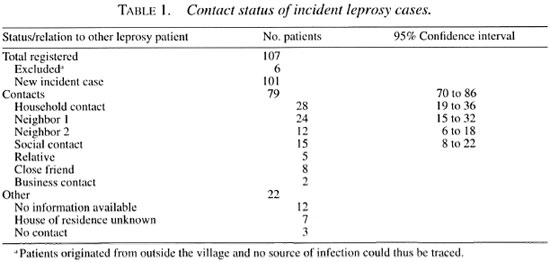
Subsequently, we traced the possible contacts between the incident cases and grouped them into the categories delined in the methodology section (Table 1). According to these criteria, 28 incident cases (28% of the total of 101 incident leprosy cases) could be classified as a household contact, 24 as neighbor 1 (24%), 12 as neighbor 2 (12%), and 15 as other contacts (14.9%); from 15 patients (14.9%) not enough information was available to be able to classify them with certainty. Of all 101 incident cases, 79 (79%) could thus be connected in place and time to a previously diagnosed leprosy patient.
In Figure 1 we have highlighted one cluster of patients showing, as an example, the occurrence of incident patients in time. In 1971 a MB patient was detected; he was treated with dapsone monotherapy but since he was not compliant with his treatment, he was re-treated with MDT from 1983 onward. In 1973 his daughter living in the same house was diagnosed with leprosy. In 1980, 1982 and 1983 incident cases were reported in neighboring houses. In 1988 two new cases were diagnosed in one of these neighboring houses and 6 years later, in 1996, another incident leprosy case was found in the cluster.
The median span of time between the registration of the primary and secondary case was 3 years (Fig. 2). Within l year after the registration of the primary case, 25% of the secondary cases were detected. This figure increased to 85% after 5 years and 95% after 6 years.
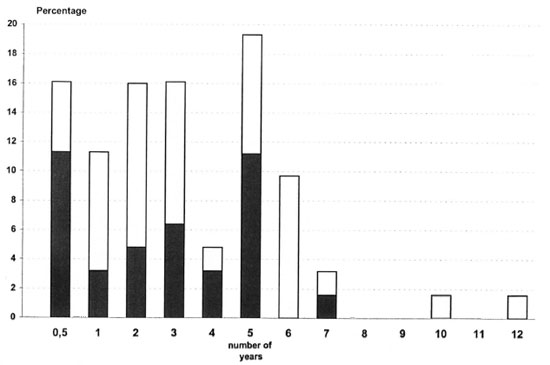
Fig . 2. Time in years between registration of index cases and new leprosy cases among neighbors and house-hold contacts.  = neighbor contacts;
= neighbor contacts;  = household contacts.
= household contacts.
In order to explore whether the mode of case detection favored the detection of new patients who were household members or neighbors of existing patients, the methods of case findings were compared with the categories of contacts status as defined in our study. Although active case detection identified a slightly higher, but not significant, number of household contacts than passive case detection [36% (13/36) versus 26% (15/58)], active case detection activities did not detect more neighbor contacts than passive case finding [31% (11/36) v.v 43% (25/58)]. Overall, no relation could be detected between the mode of detection and contact status (chi-squared, p = 0.42), excluding a possible bias toward the detection of patients in the neighborhood of existing patients through active case-finding activities of the leprosy control program.
The risk for developing leprosy for each category of contact of a patient was calculated as explained in the Materials and Methods section and is presented in Table 2. The follow up of a 6-year risk period for houses in which a MB patient was living yielded 227 household-risk-years. During these years a total of 18 patients originated from these households, giving a basic risk of 7.9 per 100 house years at risk. Similarly, there were seven patients with a PB primary case and there were 230 house years at risk for PB households, giving a risk of 3.0 per 100 house years at risk. The risk for the development of leprosy for MB household members is thus 2.6 times higher than for PB household members. The assumption here is that the average compositions of a household in the different categories are similar.
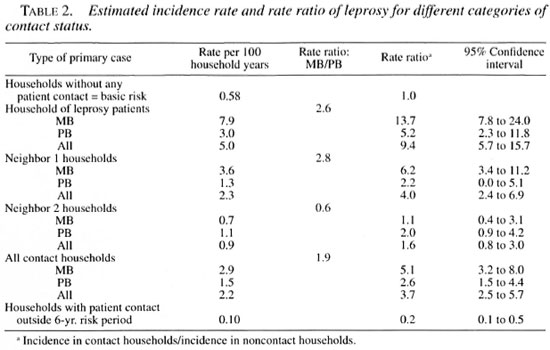
The risk for developing leprosy in neighboring houses of leprosy patients was lower overall than for the households of leprosy patients. The risk for leprosy in houses neighboring a MB patient was 2.8 higher than the risk for neighboring houses of a PB patient, which is similar to the increased risk of leprosy for persons in MB households compared to PB households.
Subsequently, we compared the risk for household contacts and for neighbor contacts against a basic risk in the village for developing leprosy. This basic risk is taken as the risk to become an incident patient in those households in which no patient had ever been living. The results of these calculations, presented in Table 2, show that the risk of leprosy is highest for household members of MB primary cases. This risk is 13.7 times more than the basic risk, followed by the risk for neighbors of MB households and household contacts of PB patients. It is evident that both the type of the source and the distance to the source are factors that determine the risk for disease.
The fact that MB patients are clearly an important source for spreading the disease raises the question of whether this applies to all MB patients or not. How many MB and PB patients actually can be implicated as an index case?
Of the 45 MB patients, 24 (53%) were the primary case of a new case of leprosy (household or neighbor), and 13 of these 24 patients (54%) generated two or three new cases in or around the household (Table 3). Of the 49 PB patients, 18 (37%) were identified as a primary case of a new case of leprosy, and four of them (22%) generated a second new case in or around the household. Thus, more MB than PB patients (30% versus 8%) could be incriminated as an index case for more than one person. A chi-squared of trend showed that MB patients were 1.16 times more likely than PB patients to generate one new case, but they were 4.8 times more likely than PB patients to be the primary case for more than one person (chi-squared of trend, p = 0.012).
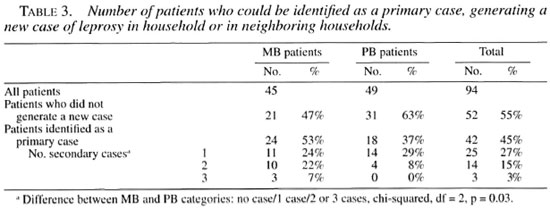
An additional four MB and one PB patients were the primary case for only social contacts, bringing the total number of patients who acted as a source of leprosy to 62% of the MB patients and 39% of the PB patients.
DISCUSSION
Leprosy is an infectious disease in which humans are considered the only source of infection. This is evidenced by the fact that persons living in the same household as leprosy patients have an increased risk of developing the disease (1,3-5). Still, the majority of incident cases cannot be related to other leprosy patients. Patients are often not aware of any previous contact, likely due to the long incubation period of the disease. Also, household contact is often the type of contact defined, both in leprosy control programs and in research. Here, we report that by expanding the criteria of contact status beyond that of households, the majority of incident leprosy patients can be related to a previous case of leprosy.
Of the 101 incident leprosy cases over 25 years, 28% could be classified as household contacts, which is higher than the 15% recently reported from a longitudinal study conducted in Malawi (3). In that study it was believed that 15% was a considerable underestimate. Also, that study left open the question of what kind of contact led to the remaining 70% or so incident leprosy cases.
By extending the criteria for contact from household to neighbor status, we could identify another 36% (N = 36) of the incident cases as being contacts. Thus, a total of 63% of the incident cases could be recognized as contacts solely on the basis of their residence. The current policy of leprosy control programs is to examine household members, since to date they were the only group known to be at high risk of developing leprosy. Our data suggest that by including neighbors into the high-risk group, the case detection rate could approximately be doubled.
Most contacts (95%) developed leprosy within a period of 6 years following the registration of the primary case. If, in addition to household and neighbor contact, social contact was also considered, we could connect another 15% of all incident cases, making the total come to over 78% which could be connected in place and time to a prior diagnosed leprosy patient. This percentage is likely to be even higher considering that no information on contact status was available for the majority of the remaining incident cases.
Since this study is a retrospective study, it was not possible to select and interview a comparable control group and to compare this group with the cases we have studied. For the establishment of the contact status on the basis of residence (household or neighbor), which is the major finding in this study, the facts on residence and time of registration were collected independently of the contact status which was only determined afterward during the analysis of the data.
On the other hand, the number of patients with contact could be inflated by case detection activities of the leprosy control program: new patients are more likely to be detected in the surroundings of existing patients. This can be due to an increased awareness of the surroundings to leprosy or due to the fact that the case-detection activities are focused around the patients. In Tumaluntung, several active case-detection surveys have been carried out which could very well favor the detection of household or neighbor contacts as patients. However, the contact status of patients detected in such active surveys appeared not to be different from the contact status of patients who were identified by passive case detection.
This study has been performed in a social setting of a small Indonesian village with a fairly equal distribution of houses unlike the scattered compounds one can find in certain African (rural) areas. However, it is very likely that the dynamics of the transmission are based on the same mechanism which is also suggested by the fact that household contacts are identified as having a risk factor for leprosy in very different countries (2,3). The study in Malawi (3) shows that transmission most likely occurs under conditions of closest contact, giving the highest risk for developing leprosy to those who share the same bedroom, followed by those who were living in the same household. Our data are in concordance with the theory that risk of leprosy infection is inversely related to the distance (or degree of contact) to a primary case. This places household contacts most at risk and makes neighbors the most at risk group.
We have attempted to estimate the risk for developing leprosy by calculating the number of new cases per household-years at risk in each category of contact. The most reliable information from these figures can be obtained by comparing these risks for each category.
The results showed that households of MB patients had the highest risk for developing leprosy; they were 13.7 times more likely to develop leprosy than were households without any contact with a leprosy patient. Households of PB patients were 5.2 times more at risk. However, the risk for neighbors of MB patients was similar to the risk for households of PB patients, indicating that both the type of leprosy of the primary case and the distance to the primary case are determining factors.
The above risks are only true under the assumption that the average household size is equal for all categories. In spite of the crude nature of these calculations, the results are such that they fit the concept that MB cases are the group most responsible for transmission of the disease. However, not all MB patients will transmit the disease. In our study 53% of the MB patients and 37% of the PB patients could be identified as the index case or primary case for a patient in the household or in the neighbor households. The increased infectiousness of MB patients in general is also manifested by the fact that more MB than PB patients (30% versus 8%) could be incriminated as a primary case for two or more new leprosy patients. In total, 54% (13/24) of the MB index patients could be linked to two or more secondary cases, while this was the case only for 22% (4/18) of the PB index cases.
Contact status in this study was defined in operational terms and thus does not necessarily reflect the dynamics of transmission. This means that the primary case is not by definition the source of infection for the secondary case; both could have had a common index case obscured by differences in incubation time. Due to the long incubation time of MB leprosy, it is not unlikely that a PB patient, identified as a primary case for a MB patient, was not the source of transmission but that the leprosy was contracted from an earlier contact with a MB case in the neighborhood of the PB patient. The results of this study might, therefore, even underestimate the infectiousness of the MB patients.
Leprosy control programs face the problem of many leprosy cases remaining undetected. This is one of the main reasons why the so-called leprosy elimination campaigns (LEC) have been initiated (6). Indeed, in this way many previously undetected patients have been discovered already; e.g., in Indonesia a 4-month campaign in West Java identified 1142 new patients, more than 4.7 times the total number of new cases otherwise notified in one year (6). However, the transmission which took place before the LECs will result in more new cases to come, years after the completion of these campaigns and likely also years after the elimination goal has been reached (7). Thus, it will remain important for leprosy control programs to detect new patients as early as possible and to do so in an effective and sustainable manner. The results of our study show that contact with a leprosy patient is the major determinant in incident leprosy, whereby the type of contact is not limited to household relationships but also includes neighbor and social relationships. This concept shows similarities with the "stone-inthe-pond" principle describing tuberculosis transmission in concentric circles around a patient. This principle could be translated into a valuable and sustainable tool for leprosy control programs and elimination campaigns by focusing case detection and health promotion activities not only on household contacts but also on at least neighbors of leprosy cases.
Acknowledgment. We acknowledge with appreciation the financial support for this study received from the Netherlands Leprosy Relief Association and the Gastmann-Wichers Foundation. We thank Dr. Frankie Loprang, Head CDC North Sulawesi, for his enthusiastic support and Dr. Agnes Kwenang, Mr. Romi and Mr. Marwani for their help in the village. We appreciate the cooperation and assistance of the local leprosy control program, in particular Mr. Saleh Taawa and Ms Ida Runtukahu. We thank all of the inhabitants of Tumaluntung for their cooperation.
REFERENCES
1. Douglas, J.. Cellona, R., Abalos, R., Fajardo, T., Ki.atser, P. and Walsh, G. Prospective study on the early detection of leprosy in household contacts in Cebu. Abstracts, 96th General Meeting of the American Society for Microbiology, 1996. p. 129.
2. Doull, S. A.. Guinto, R. S. and Rodriguez, J. N. The incidence of leprosy in Cordova and Talisay, Cebu. Philippines. Int. J. Lepr. 10(1942)107-131.
3. Fine;. P. E.M., Sterne, J. A. C., Ponnighaus, J. M., Bliss, L., Saui., J.. Chihana, A., Munthali, M. and Warndorff , D. K. Household and dwelling contact as risk factors for leprosy in northern Malawi. Am. J. Epidemiol. 146(1997)91-102.
4. Leprosy beyond the year 2000. (Editorial) Lancet 350(1997)1717.
5. van Beers, S.M.. de Wit, M. Y. L. and Klaster, P. R. The epidemiology of Mycobacterium leprae : recent insight. FEMS Microbiol. Lett. 136(1996)221-230.
6. World Health Organization. Leprosy elimination campaigns-reaching every patient in every village. Wkly. Epidemiol. Rec. 72(1997)205-212.
7. World Health Organization. Progress towards leprosy elimination. Wkly. Epidemiol. Rec. 72(1997)165-172.
1. Ph.D., Department of Biomedical Research, Royal Tropical Institute, Meibergdreef 39, 1105 AZ Amsterdam, The Netherlands..
2. Ph.D., Department of Biomedical Research, Royal Tropical Institute, Meibergdreef 39, 1105 AZ Amsterdam, The Netherlands.
3. M.D., Ph.D., Department of Microbiology, Hasanuddin University, Ujung Pan-dang, South Sulawesi, Indonesia.
Reprint requests to Dr. Paul R. Klatser at the above address. Tel: 31-20-566-5441; email: P.Klatser@kit.nl
Received for puhlication on 28 April 1998.
Accepted for publication in revised forro on 23 April1999.