- Volume 67 , Number 2
- Page: 133–42
Lymphoproliferative responses of leprosy patients and healthy controls to nitrocellulose-bound m. bound m. leprae antigens
ABSTRACT
The lymphoproliferative responses of 51 leprosy patients and 11 healthy contacts were analyzed using the nitrocellulose-bound specific antigen fractions from the cell-free extract of Mycobacterium leprae. The main proliferation-inducing fraction for peripheral blood mononuclear cells of the healthy contacts was found to be the Fraction II, bearing antigens in the range of 66-45 kDa. However, this fraction failed to induce lymphoproliferation in the leprosy patients, unlike healthy contacts (p <0.032). The number of responders as well as the strength of the responses to 66-45 kDa proteins were found to be low in the leprosy patients compared to the healthy contacts. Further, preliminary analysis with the subfractions of Fraction II produced a similar pattern, suggesting that the immune response to the antigens in the range of 66-45 kDa M. leprae proteins remains suppressed in subjects with clinical signs and symptoms of the disease.RÉSUMÉ
La réponse au test de stimulation lymphocytaire de 51 patients atteints de lèpre et de 11 personnes asymptomatiques en contact avec ces patients a été analysée en utilisant des fractions antigéniques spécifiques provenant d'extraits acellulaires de Mycobacterium leprae, fixées sur membrane de nitrocellulose. La fraction principale, induisant une prolifération des cellules mononuclées du sang périphérique chez les personnes contactes, fut déterminée être la 2ème fraction (fraction II), contenant des antigènes de l'ordre de 66-45 kilodaltons (kDa). Cependant, cette même fraction n'a pas induit de prolifération lymphocytaire parmi les patients lépreux, à la différence des sujets contacts (p <0.032). Le nombre de cas ayant une réponse, ainsi que l'intensité de celle-ci vis-à-vis des protéines de 66-45 kDa, étaient basse chez les patients hanséniens en comparaison avec les sujets contacts. De plus, les résultats d'une analyse préliminaire utilisant les sous-fractions de la fraction II sont similaires, suggérant que la réaction immunitaire contre les antigènes protéiques de M. leprae de l'ordre de 66 à 45 kDa reste inhibée chez des individus présentant des signes et symptômes de cette maladie.RESUMEN
Se analizaron las respuestas proliferativas de los linfocitos de 51 pacientes con lepra y de 11 contactos sanos utilizando diferentes fracciones antigénicas de Mycobacterium leprae unidas a nitrocclulosa. En los contactos sanos, la principal fracción inductora de linfoproliferación fue la fracción II con antígenos en el rango de 66-45 kDa. A diferencia de lo que ocurrió en los contactos sanos, esta fracción no indujo linfoproliferación en los pacientes con lepra (p <0.032). Así mismo, el número de respondedores y la intensidad de las respuestas a las proteínas de 66-45 kDa fueron más bajos en los pacientes con lepra que en los contactos sanos. Adcmás, los experimentos preliminares con subfracciones de Ia fracción II dieron resultados similares, sugiriendo que la respuesta inmune a los antígenos de 66-45 kDa de M. leprae se encuentra suprimida en los sujetos con signos y sintomas clínicos de la enfermedad.
The most intriguing aspect of leprosy is the tremendous range of cell-mediated immune response to the same causative organism, i.e., Mycobacterium leprae (23). The spectrum of the immune response is reflected either as strong T-cell reactivity paralleled by the resistant phenotype with few lesions and bacilli in paucibacillary (PB) patients or as the anergy resulting in multiple lesions and bacilli in multibacillary (MB) patients (22). A great number of strides have been made to unearth this mystery but without much success. Most of these studies have revealed differences at the immunological level (i.e., type of T-cell sub-set, cytokine secretion, etc.) between these two types of leprosy patients (11, 15, 16, 25). However, it remains to be established unequivocally whether the differential immune response in the exposed healthy contacts and the patients displaying different clinical forms of the disease is induced by different sets of M. leprae antigens. In the past, few attempts have been made to characterize the antigens which confer protective and pathologic immunity in PB and MB patients, respectively (3, 4, 10, 19, 26), but a considerable amount of heterogeneity is evident in these reports. The present study is an attempt to analyze whether the proliferative responses of leprosy patients and healthy contacts are directed against different sets of M. leprae antigens.
Here we report that unlike the healthy contacts, most of the Indian PB as well as MB patients did not respond in vitro to 66-45 kDa M. leprae antigens, suggesting that an in vitro immune response to one of these antigens might be critical in deciding the final outcome of M. leprae infection.
MATERIALS AND METHODS
Samples. Fifty-one leprosy patients included in this study were attending the routine diagnosis clinic at the All India Institute of Medical Sciences, New Delhi, India. Clinical diagnosis and classification were made by experienced dermatologists in accordance with the established routine classification (6, 23). A bacterial index (BI) was determined for each patient. A skin biopsy was taken and the patients were assigned to one of two groups: a) PB = indeterminate (ID = 3), borderline tuberculoid (BT = 25), borderline tuberculoid/borderline borderline (BT/BB - 10) and b) MB = borderline borderline (BB = 4), borderline borderline/borderline lepromatous (BB/BL = 4), borderline lepromatous/lepromatous leprosy and lepromatous leprosy (BL/LL, LL = 5). The BT/BB cases were assigned on the basis of their symptomatic tendency to shift toward BB as per their clinical records followed over the period of time. Out of 51 patients, 18 were untreated while the rest were on treatment for less than 1 year. Healthy contacts (HC =11) included in the study were household contacts and the first-degree relatives of the patients.
Preparation of antigens. M. leprae whole cell free extract (WCFE) was obtained in lyophilized form as a kind gift from Dr. R. J. W. Rees (NIMR, London), through the Immunology of Leprosy (IMMLEP) project under the WHO/ UNDP/World Bank Special Programme for Research Training in Tropical Diseases. Purifled protein derivative (PPD) of M. tuberculosis was obtained from Span Diagnostics, India.
Fractionation of WCFE. Three-hundred micrograms of WCFE and PPD were fractionated on the basis of their molecular weight using the Tricine SDS procedure (27). The WCFE as well as PPD antigens were heated at 100°C for 5 min and simultaneously separated by SDS-PAGE in identical sized wells. In one lane, no sample was loaded. The molecular weight markers (mw SDS, 17S; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) were also run as references for the determination of molecular mass. The gel was run for 16 hr at 15V. The protein blot was removed and rinsed with water. A part of the blot including the molecular weight markers was stained with Ponceau-S stain for an accurate localization of the molecular weight markers (Fig. 1). The unstained blot was cut with a sterile scalpel into different fractions on the basis of their molecular weights: FI (>66 kDa), FII (66-45 kDa), FIII (45-36 kDa), FIV (36-29 kDa) (Fig. 1). A fraction of nitrocellulose from a lane without any protein was used as the control. All test fractions and the control fraction contained the same amount of nitrocellulose. The test fractions (5x3 mm) containing the WCFE/PPD proteins and the control fraction (5x3 mm) were converted into fine antigen-bearing particles (1) by dissolving them into 2 ml of sterile dimethylsulfoxide for 1 hr at room temperature. The nitrocellulose particles were precipitated by the addition of carbonate/bicarbonate buffer (0.05 M, pH 9.6) with continuous stirring in a vortex mixer. The particles were washed three times with RPMI-1640 by centrifuging at 12,000 rpm for 10 min. The particles were finally suspended in 1 ml of sterile RPMI-1640, divided in aliquots, and stored at -70°C. Subfractions of each fraction (FI, FII and FIII ) were prepared by the same protocol using the same batch of cell-free extract.
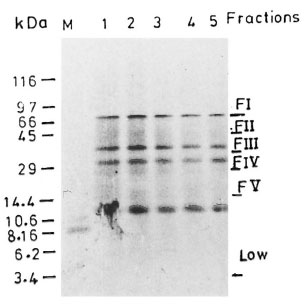
Fig . 1. Fractionation of whole cell free extract (WCFE) of M. leprae in 10 % tricine SDS-PAGE: 300 µg of protein sample run at 80V were visualized by Coomassie blue staining (lanes 1-5). Fractions I-V represent distinct protein components of M. leprae ex-tract; FI = >66 kDa, FII = 66-45 kDa, FIII = 45-36kDa. Data obtained with M. leprae proteins of molecular weight 29 kDa (FV) are not included in this study. Less intensely stained bands are indescernible in the final picture.
Lymphocyte proliferation assays. Fresh peripheral blood mononuclear cells (PMBC) were isolated from the blood samples (2). Cells were suspended in RPMI-1640 supplemented with 10% fetal calf serum (FCS) and plated (1 x l05/well) in triplicate in flat-bottom, half-area microtiter wells (Costar Corp., Cambridge, Massachusetts, U.S.A.) with or without antigens. WCFE (10 µg/ml) and fractions of nitrocellulose-bound cell-free extract (nbCFE) of M. leprae were used as antigens. Response to PHA (10 µg/ml) was taken as a positive control. The cultures were incubated at 37°C in a humidified 5% CO2, air mixture for 5 days. Cultures were pulsed overnight with 0.5 µCi of tritiated thymidine (BARC- Bombay) per well and harvested on day 6 onto glass fiber filters with an automated cell harvester (PHD). Incorporation of 3H-thymidine was measured by liquid scintillation spectroscopy.
Statistical analysis. The mean count per minute (cpm) of each triplicate culture without antigen was subtracted from the Δcpm of stimulated culture, and the data were expressed as Δcpm. Δcpm were found to be generally low as the cells were plated in half-area microtiter wells for lymphopro- liferation assays. The mean counts for control cultures without WCFE were 1640 cpm; for control cultures with nitrocellulose without any bound protein, 980 cpm. A Δcpm of >2000 cpm and a stimulation index (SI = cpm of stimulated culture/cpm of culture without antigen) of >2 was considered to be a positive response. The chi-squared test was used to determine the significance of the difference in the number of responded to different antigens between different clinical groups. Comparison of strengths of lymphoproliferative responses between groups of individuals was made by the Mann-Whitney test.
In order to rule out the possibility of a low response (as indicated by a low Δcpm) to unfractionated WCFE, especially in the HC and PB groups due to technical artifacts, the data were analyzed against their response to PHA (data not shown). A significantly high response to PHA in these groups ruled out the possibility of poor cell viability. Moreover, the lymphoproliferative assays to different antigens (WCFE/fractions) were carried out simultaneously under similar conditions.
Optimal stimulatory activity and specificity for each fraction were established as a pilot experiment using different dilutions of nitrocellulose-bound M. leprae and M. tuberculosis antigens (Fig. 2). Nitrocellulose-bound CFE of M. leprae antigens as stock (Fig. 2a) appeared optimal as compared to 1:10 and 1:100 dilutions (Figs. 2b, c) and, subsequently, stock antigens were used in the test experiments for stimulating lymphocytes from patients and healthy contacts. Figures 2d and 2e show the inability of nitrocellulose-bound purified protein derivative (nbPPD) to elicit in vitro proliferation of PBMC of the patient, who responded positively to the nitrocellulose-bound M. leprae fractions FII and FIII (Fig. 2a). Thus, the presence of any contaminant, either running along with M. leprae in the gel or in other reagents, which could have elicited the nonspecific stimulation or inhibition of lymphoproliferation of the PBMC of the donors was ruled out. When tested with PBMC of a BB patient, the nbPPD fractions evoked a positive lymphoproliferation (Fig. 2f). However, the pattern of thelymphoproliferative response was entirelydifferent. Fraction IV of nbPPD was found stimulatory for PBMC from this patient, who did not respond to any of the nbCFE fractions of M. leprae .
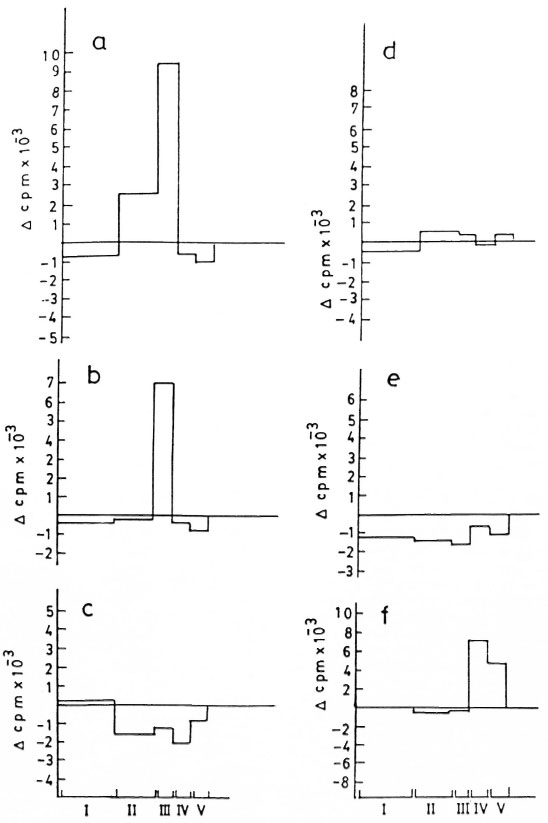
Fig . 2. Lymphoproliferative responses of a borderline tuherculoid (13T) patient to fractions of nitrocellulose-bound cell-free extract (nhCFE) of M. leprae tested over a range of dilutions a) stock, h) 1:10 and c) 1:100, andto the fractions of nitrocellulose-hound purified protein derivative (nhPPD) of M. tuberculosis tested at d) stockand e) 1:10 dilutions, f) PBMC response of a horderline horderline (BB) patient to nhPPD.
RESULTS
In vitro lymphoproliferation to WCFE of M. leprae . A significant number of the PB patients responded to WCFE as compared to MB patients (p <0.05; The Table). The magnitude of response of the PBMC of healthy contacts and patients with different clinical forms of leprosy to the WCFE of M. leprae is shown in Figure 3. The strengths of WCFE-induced lymphoproliferation decreased along the leprosy spectrum from PB to MB patients. However, within all three groups a wide variation in the strength of in vitro lymphoproliferation was observed. The lymphoproliferative responses of HCs varied from -762 to 14,868 cpm. In PB patients, the response to WCFE ranged from -3174 to 11,900 cpm and in MB patients from -2376 to 3158 cpm.
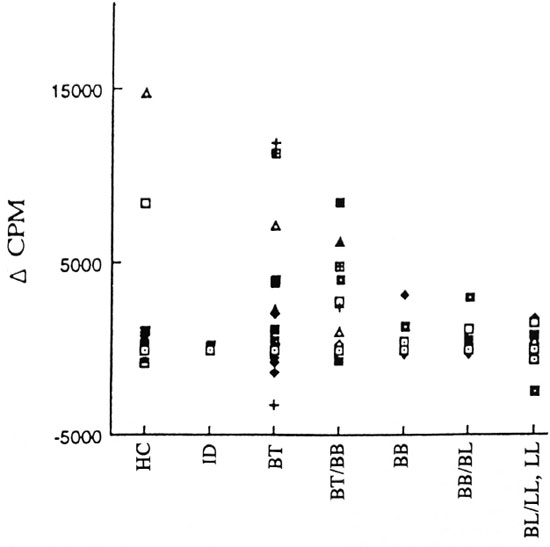
Fig . 3. Strength of in vitro proliferation of PBMC to whole cell-free extract (WCFE) in patients with different clinical fornis of leprosy: indeterminate (ID), borderline tuberculoid (BT), horderline tuberculoid/horderline horderline (BT/BB) of PB leprosy and borderline horderline (BB), horderline horderline/horderline lep-romatous (BB/BL) and borderline lepromatous leprosy, lepromatous leprosy (BL/LL,LL) of MB leprosy patientsand healthy contacts (HC).
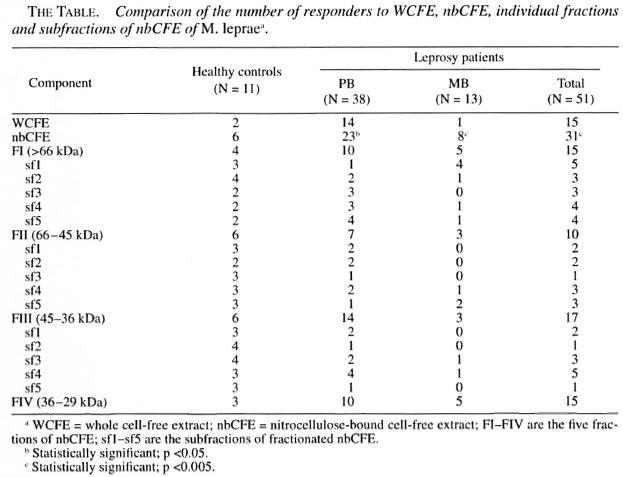
In vitro lymphoproliferation to nbCFE of M. leprae . When compared for the number of responders to the nitrocellulose bound M. leprae antigens (irrespective of the fraction to which they responded) and unfractionated M. leprae antigens within each group, a significant number of subjects responded to fractionated antigens as compared to WCFE. This difference was found to be more significant within the MB group (p <0.005) compared to PB patients and HCs (The Table).
When compared for the number of responders to the specific fractions between different groups of subjects, the number of responders to FI (>66 kDa) and FIII (45-36 kDa) were found to be higher in the HCs compared to the patients (The Table). However, this difference failed to reach a statistical significance. Also, no significant difference was found in the number of responders to the FIV (36-29 kDa) fraction between the HCs and the patients
The most significant difference in the lymphoproliferative responses of the HCs and the patients was observed against the FII (66-45 kDa) fraction (Fig. 4). This fraction was recognized by a higher proportion of HCs compared to the leprosy patients (p <0.025; The Table). Not only the number of responders but also the strengths of the lymphoproliferative responses of the HCs to FII (average cpm = 7697) were found to be significantly higher compared to the leprosy patients (p <0.032; average cpm - 1759), including the PB (p <0.050; average cpm = 1367) as well as MB patients (p <0.027; average cpm = 2151).
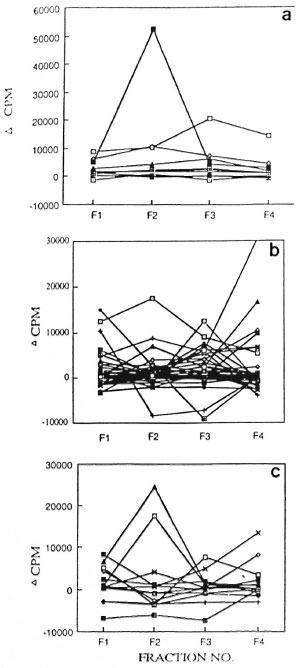
Fig . 4. Strength of proliferative responses to fractions I-IV of nbCFE in a) HC, b) PB leprosy, and c) MB subjects. Proliferative responses from sante subjects were evaluated against fractions I-IV shown as Fl, F2, F3 and F4.
Responses to subtractions of FI, FII and FIII. Thirty-two leprosy patients (23 PB and 9 MB) and 11 HCs were further probed for their lymphoproliferative responses to the subtractions of fractions I, II and III. Each fraction was subdivided into five subfractions (sfl, sf2, sf3, sf4 and sf5) in order to further dissect the antigenic moieties which could discriminate the lymphoproliferative responses of the leprosy patients and the HCs.
Response to the subfractions of Fraction I: The PB patients failed to respond to the sfl (>100 kDa) of FI; only 1 out of 23 (4%) PB patients responded to the sfl of FI. Interestingly, this patient, clinically diagnosed as BB (MB), showed lesions which were histopathologically of the BT (PB) type. In the MB patients 4 out of 9 (44.4%) showed a positive response to this subfraction. Thus, a significant difference was found in the number of responders to sfl of FI in PB and MB patients (x2 = 7.8; p <0.005; The Table). The only patient (PB) who showed a positive response to this subfraction was undergoing a type 1 reaction.
Response to the subtractions of FII: Almost every subtraction of FII (sfl, sf2, sf3, sf4 and sf5) was capable of stimulating lymphoproliferation in the HCs. However, the difference in the number of responders to sf3 (55-60 kDa) was found to be statistically significant between the HCs and leprosy patients (x2= 5.65; p <0.025; The Table). The only patient (PB) who showed a positive response to this subfraction was undergoing a type 1 reaction.
Response to the subfractions of FIII : The subfraction sf2 of FIII (43-40 kDa) was recognized more frequently by the HCs compared to the PB patients (x2 = 5.95 p <0.025; The Table).
DISCUSSION
Appropriate cell-mediated immunity is a prerequisite for protection against M. leprae infection. In the past, few attempts have been made to identify specific or crossreactive mycobacterial antigens which could confer protective or pathologic immunity in resistant or susceptible hosts, respectively, so that these antigens could be used as skintest reagents to measure delayed-type hypersensitivity, as vaccines, or as targets of immunoregulation (3). The present study was an attempt to identify a set of antigens which could discriminate the immune responses of leprosy patients and healthy contacts. The PBMC of healthy contacts and patients were stimulated with both unfractionated as well as nitrocellulose-bound fractions of M. leprae proteins. The data were analyzed for the number of responders as well as the strengths of lymphoproliferation in the different groups.
Results of in vitro proliferative tests to WCFE unfractionated M. leprae antigens of PBMC from leprosy patients across the disease spectrum established a correlation of cell-mediated immunity with the clinical and histopathologic picture in the MB patients as indicated by their negative proliferative responses. Interestingly, a significant number of the PB patients also did not respond in vitro to WCFE. This observation did not correspond to their histological pictures in skin biopsies which showed evidence for cell-mediated immunity. This anergy to WCFE in the PB group did not seem to result from technical artifacts such as nonviability of PBMC, nonoptimal concentration of the antigen, treatment profile, duration of disease (data not shown). This led to the assumption that even within one category (i.e., paucibacillary) defined by the clinical and histopathologic parameters there could exist subgroups that differ in their immune response with respect to the type of antigen they recognize. The presence of such subgroups within lepromatous leprosy has already been documented by some workers (8-21)
It was found in the present study that a significant number of WCFE nonresponder leprosy patients responded to M. leprae antigens when their PBMC were challenged with fractionated antigens. This finding negated the previous observations which could not detect any enhancement of response to fractionated antigens (4-6). Enhancement of in vitro lymphoproliferative responses in MB patients on challenge with fractionated antigens in the present study confirmed that there is no lack of M. leprae - reactive lymphocytes in the peripheral circulation of lepromatous leprosy patients (14). However, the inability of such cells to recognize M. leprae antigens in vivo and in vitro conditions when presented as a mixture of proteins suggested the probability of the presence of some antigens in unfractionated M. leprae antigens which could mask the proliferation of the M. leprae -reactive cells to the antigens of M. leprae .
Several mycobacterial antigens have been discovered as dominant T- and B-cell antigens in the responses of healthy as well as mycobacterial disease affected individuals (20,30). Using synthetic peptides and recombinant deletion mutants analysis, well-defined T-cell epitopes have been mapped on mycobacterial heat shock proteins such as 65 kDa (13,28), 70 kDa (l7,18) and 10 kDa (9). T-cell epitope mapping has also been conducted on the fibronectin-binding antigen 85 (9) and 16 kDa (29) proteins. The B-cell epitopes have been shown to exist on a variety of proteins, such as 10 kDa heat shock protein (24), 18 kDa (7), 44.3 kDa (l2), and 9 kDa (l2) and phosphoglycolipid I (24), in various mycobacterial species.
In this study of the response to individual groups of M. leprae antigens, in nitrocellulose-bound form, the antigens in the molecular weight range of 66-45 kDa (FII) were found to be recognized more by the healthy contacts than by the leprosy patients. This finding is in confirmation with a previous report indicating that PBMC of the healthy contacts of patients in a leprosy-endemic country showed prominent responses to M. leprae in the range of 45-66 kDa (3). Similar observations were made in another study using two-dimensional PAGE separated antigens where responses of the contacts were directed to protein fractions with a molecular weight of 50-80 kDa (5).
This anergy to 66-45 kDa M. leprae could not have arisen because of the differences in the relative number or concentration of proteins in each fraction (as evident from Fig. 1). Moreover, the same batch of M. leprae cell-free extract was used throughout the study for patients and controls.
The failure of 66-45 kDa M. leprae proteins to evoke lymphoproiiferation in a significant number of PB patients is intriguing. It has been suggested that during different stages of the disease different sets of antigens are recognized by the patients (3). It is likely that during the subclinical stage of the disease the 66-45 kDa antigens are recognized and, as the disease progresses, other sets of antigens evoke responses (helper/suppressor). This study also suggests that the clearance of bacilli in PB patients is probably triggered by an exaggerated immune response to antigens other than the 66-45 kDa antigens. The other possibility is the presence of some epitopes in the 66-45 kDa antigens which initiates a cascade of events in the cell-mediated immune response and finally prove to be protective in nature. Our preliminary experiment conducted on the PBMC of the leprosy patients and healthy contacts using the subfractions of FII has identified some proteins in the range of 55-60 kDa which were recognized differentially by the healthy contacts and the leprosy patients.
Further analysis using FII and the other subfractions, sf 1 of FI and sf2 of FIII , on a larger sample size could prove worthwhile in the identification of M. leprae antigens which evoke a differential immune response in exposed healthy contacts and leprosy patients.
REFERENCES
1. Abou-Zeid, C, Fll ey, E., Stelle, J. and Rook, G. A. W. A simple method for using antigens separated by Polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen bearing particles. Immunol. Lett. 98(1987)5-10.
2. Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21(1968)77-79.
3. Converse, P. J., Ottenhofe, T. H. M., Gebre, N., Ehrenberg, J. P. and Kiessling, R. Cellular, Humoral and gamma interferon response to Mycobacterium leprae and BCG antigens in healthy individuals exposed to leprosy. Scand. J. Immunol. 27(1988)515-525.
4. Filley, E., Abou-zeid, C.. Waters, M. and Rook, G. The use of antigen-bearing nitrocellulose particles derived from Western blots to study proliferative responses to 27 antigenic fractions from Mycobacterium leprae in patients and controls. Immunology 67(1989)75-80.
5. Gulle, H., Schoel, B., Chiplunkar, S., Gangal, S., Deo, M. G. and Kaufmann, S. H. E. T cell responses of leprosy patients and healthy contact towards separated protein antigens of Mycobacterium leprae . Int. J. Lepr. 60(1992)44-53.
6. Hastings, R. C. (ed.). Leprosy 2nd edn. Singapore: Churchill Livingstone, Longman 1994.
7. Hussain, R., Menz, B., Dockrell, H. M. and Chiang, T. J. Recognition of Mycobacterium leprae recombinant 18 kDa epitopes by IgG subclasses in leprosy. Immunology 84(1995)290-297.
8. Kaplan, G., Weinstein, D.. Steinman, R. M., Levis, W. R., Elvers, V., Pattarroyo, M. E. and Cohn, Z. A. Analysis of in vitro T cell unresponsiveness in lepromatous leprosy. J. Exp. Med. 162(1985)917-929.
9. Launois, P., N'Diaye, M. N., Cartel, J. L., Mane, I., Drowart, A., van Vooren, J. P., Sarthou, J. L. and Huygen, K. Fibronectin-binding antigen 85 and the 10 kDa Gro Es-related hsp are the predominant TH-1 response inducers in leprosy contacts. Infect. Immun. 63(1995)88-93.
10. Lee, S. P., Stoker, N. G., Grant, K. A., Handzel, Z. T., Hussain, R., McAdam, K. P. W. J. and Dockrell. II. M. Cellular immune responses of leprosy contacts to fractionated Mycobacterium leprae antignes. Infect. Immun. 57(1989)2475-2480.
11. Modlin, R. L., Gebhard, J. F, Taylor, C. R. and Rea, T. H. In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin. Exp. Immunol. 53(1989)171-174.
12. Mutuaria, L. M., Moreno, W. and Raymond, M. Analysis of culture filtrate and cell wall-associated antigens of Mycobacterium paratuberculosis with monoclonal antibodies. Infect. Immun. 65(1997)387-394.
13. Muris, T., Cornelisse, Y. E., Datema, G., van den Elsen, P. J., Ottenhoee, T. H. and de Vries, R. R. P. Definition of a human suppressor T-cell epitope. Proc. Natl. Acad. Sci. U.S.A. 91(1994) 9456-9460.
14. Nath, I., Sathish, M., Ayaraman, T., Biiutani, L. K. and Siiarma, A. K. Evidence for the presence of M. leprae reactive T lymphocytes in patients with lepromatous leprosy Clin. Exp. Immunol. 58(1984)522-530.
15. Nathan, C. F., Kaplan, G., Levis, W. R., Nusrat, A., Witmer, M. D., Sherwin, S. A., Job, C. K., Horowitz, C. R., Steinman, R. M. and Cohn, Z. A. Local and systematic effects of intradermal recombinant interferon gamma in patients with lepromatous leprosy. N. Eng. J. Med. 315(1986)6-15.
16. Nogueira, N., Kaplan, G., Levy, E., Sarno, E. N., Kushner, P., Granelli-Piperno, A., Vieira, L., Colomer Gould V., Levis, W., Steinman, R., Yip, Y. K. and Cohn, Z. A. Detective gamma interferon production in leprosy: reversal with antigen and interleukin 2. J. Exp. Med. 158(1983)2165-2170.
17. Oitung. F, Geluk, A., Lundin, K. E., Meloen, R. H., Thole, J. E., Mustaea, A. S. and Otten-hoff, T. H. Mapping of multiple HLA class Il-restricted T-cell epitopes of the mycobacterial 70 kDa heat shock protein. Infect. Immun. 62(1994)5411-5418.
18. Oitung, F. and Lundin, K. E. Identification of mycobacterial hsp 70 reactive human T cell clones discriminating between M. tuberculosis and M. leprae . FEMS Immunol. Med. Microbiol. 20(1998)145-151.
19. Oftenhoff, T. H. M., Converse, P. J., Gebre, N, Wondimu, A., Ehkenberg, J. P. and Klessing, R. T cell responses to fractionated M. leprae antigens in leprosy; the lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur. J. Immunol. 19(1989)707-713.
20. Ottenhoff, T. H. M., Haanen, J. B. A. G., Geluk, A., Muris, T., Kaleab, B., Thole, J. E., van Schooten, W. C. A., van den Elsen, P. J. and de Vries, R. R. P. Regulation of mycobacterial heat-shock protein-reactive T cells by HLA class II molecules: lessons from Leprosy. Immunol. Rev. 121(1991)171-191.
21. Ottenhoff, T. H. M., Wondimu, A. and Reddy, N. N. B. A comparative study on the effects of rIL-4, rIL-2, rIFN-gamma, and rTNF-αlpha on specific T cell nonresponsiveness to mycobacterial antigens in lepromatous leprosy patients in vitro . Scand. J. Immunol. 31(1990)553-569.
22. Ridley, D. S. Pathology and bacteriology of early lesions in leprosy. Int. J. Lepr. 39(1971)216-224.
23. Ridlky, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)253-273.
24. Rojas, R. E., Di michllis, S. O., Sarno, E. N. and Segal-Eiras, A. IgM anti-phenolic glycolipid I and IgG anti-10 kDa heat shock protein antibodies in sera and immune complexes isolated from leprosy patients with or without erythema nodosum Icprosum and contacts. FEMS Immunol. Med. Microbiol. 19(1997)65-74.
25. Salgamk, P., Ahrams, J. S., Clayberger, C., Golstlin, H., Connil, J., Modlin, R. L. and Bloom, B. R. Different lymphokine profiles and functional subsets of human CD4+ and CD8+ T cell clones. Science 254(1991)279-281.
26. Samperio, P. M., Lamb, J., Bothamlky, G., Stanley, P., Ellis, C. and Ivanyi, J. Molecular study of the T cell repertoire in family contacts and patients with leprosy. J. Immunol. 142(1989)3599-3604.
27. Shagger, H. and Jagow, G. V. Tricine SDS-PAGE for separation of proteins in the range of 1 to 100 kDa. Analyt. Biochem. 166(1987)368-379.
28. Struyk, L., Hawes, G. E., Haanen, J. B., de Vries, R. R. P. and van den Elsen, P. J. Clonal dominance and selection for complementarity determining region 3 motifs among T lymphocytes responding to the HLA-DR3 associated Mycobacterium leprae heat shock protein 65 kDa peptitde 3-13. Hum. Immunol. 44(1995)220-227.
29. Wilkinson, R. J.. Wilkinson, K. A., De Smet, K. A., Haslov, K., Pasvol, G., Singh, M., Svarcova, I. and Ivanyi, S. Human T and B cell reactivity to the 16 kDa alpha-crystallin protein of Mycobacterium tuberculosis . Scand. J. Immunol. 48(1998)403-409.
30. Young, D. B., Lathigra, R., Hendrix, R., Sweetser, D. and Young, R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 85(1988)4267.
1. Ph.D., Human Genetics Lahoratory, Schoolof Life Sciences, Jawaharlal Nehru University, NewDelhi 110067, India. L. K. Bhutani, M.D., Departmentof Dermatology, Ali India Institute of Medical Sei-ences, New Delhi 110029, India.
2. Ph.D., Human Genetics Lahoratory, Schoolof Life Sciences, Jawaharlal Nehru University, NewDelhi 110067, India. L. K. Bhutani, M.D., Departmentof Dermatology, Ali India Institute of Medical Sei-ences, New Delhi 110029, India.
3. Ph.D., Human Genetics Lahoratory, Schoolof Life Sciences, Jawaharlal Nehru University, NewDelhi 110067, India. L. K. Bhutani, M.D., Departmentof Dermatology, Ali India Institute of Medical Sei-ences, New Delhi 110029, India.
4. Ph.D., Human Genetics Lahoratory, Schoolof Life Sciences, Jawaharlal Nehru University, NewDelhi 110067, India. L. K. Bhutani, M.D., Departmentof Dermatology, Ali India Institute of Medical Sei-ences, New Delhi 110029, India.
Reprint requests to Prof. Bamezai at the above address or FAX 91-011-616-5886 or 91-011-616-9962 or emai1: hamezai@jnuniv.ernet.in
Received for puhlication on 3 Fchruary 1998.
Accepted for publication in revised form on 19February 1999.