- Volume 67 , Number 1
- Page: 67–70
A lost talisman: catastrophic decline in yields of leprosy bacilli f rom armadillos used for vaccine production
To the Editor:
The editorial by Meyers(7) in the March 1998 issue of IJL addresses timely and important issues on the role of the armadillo in leprosy research. He states that "it was most unfortunate that deep-seated controversy surrounded research on this animal model and, as a result, the armadillo was soon largely relegated to an industrial role- namely, the manufacture of large numbers of leprosy bacilli for in vitro biochemical, immunologic, chemotherapeutic, and eventually molecular biologic studies." He also states that armadillos yield 103 leprosy bacilli each. This is a misconception. Using present production methods, yield is only 1011 to 1012 Mycobacterium leprae per animal(5). At the Gulf South Research Institute (GSRI), New Iberia, Louisiana, U.S.A., yields were as high as 1014 M. leprae per animal. This difference of 2 to 3 orders of magnitude had a profound effect on the World Health Organization (WHO) Immunology of Leprosy (IMMLEP) program, and will handicap any future programs dependent upon armadillo-derived bacilli.
As Principal Investigator on projects that produced high-yielding armadillos at GSRI and low-yielding armadillos at the Florida Institute of Technology (FIT), Melbourne, Florida, U.S.A., I am uniquely qualified to piece together the details of what happened. In late 1973 we sent 4 g of infected tissues from GSRI to Tore Godal of WHO. He estimated they contained 1011 acid-fast bacilli (AFB). This prompted him to send a 2 g sub-sample to R. J. W. Rees of the National Institute for Medical Research (NIMR), London, U.K. Rees found that they contained 1.1 × 1012 AFB per g (wet weight), and that one g of bacilli (dry weight) contained 1.4 × 1014 M. leprae . By June of 1974, Rees had found that seven tissue samples taken from four GSRI animals contained an average of 4.4 × 10" AFB/g (The Table). One armadillo, No. 5, contained enough bacilli in its liver and spleen alone for the preparation of 132,000 doses of the vaccine eventually developed by WHO. High bacterial yield was confirmed independently by Kato, et al. of the University of Montreal, Montreal, Canada, who isolated 60 mg dry weight of bacilli from 10-12 g of tissue(5).
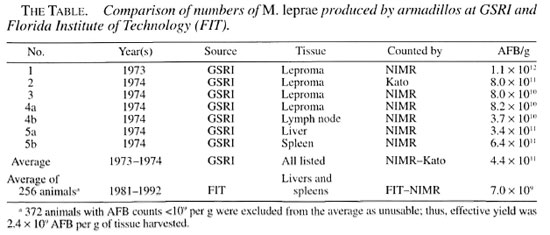
At the first meeting of the WHO Immunology of Leprosy (IMMLEP) Project Group in Geneva in November 1974, GSRI agreed to supply the IMMLEP Tissue Bank, headed by Rees, with infected armadillo livers and spleens. The first shipment was made in January 1975. In October 1975, Rees(9) reported that 323 g of tissue had yielded 492 mg (dry weight) of M. leprae, equivalent to 2.1 x 10" AFB per g. Bacterial yields from various batches of tissue differed by less than 7%. Some 300 mg of freeze-dried M. leprae had been distributed by the Bank to various investigators on behalf of IMMLEP.
In an article in Vaccine , Stewart-Tull(10) confirmed the figures published by Rees, and quoted him as saying that the average armadillo would yield 125,000 doses of vaccine, and that 150 armadillos would be needed to obtain 180 g dry weight of M. leprae . This latter statement shows beyond doubt that Rees and, consequently, WHO, expected armadillos to produce 1.7 × 1014 M. leprae each.
In a 1982 paper in Tubercule, Draper(3) bestowed the ultimate accolade on armadillos as a source of bacilli for leprosy research by writing that that they would yield as many as 1012 AFB per g, as many as might be obtained from bacteriological media though with rather more trouble. Of course, the latter has still not been accomplished. Draper based this assessment on a report issued by WHO in 1980. Hence, it must have been founded on armadillo tissues shipped to London from 1975 through 1978, when the GSRI program still existed.
In 1982, Maugh(6) writing Science stated that armadillos yielded as many as 1012- leprosy bacilli each, a sufficient number for both research and vaccine production. However, estimates from people closely associated with the WHO program had already begun to decline. In 1980, Rees told an Associated Press reporter that each armadillo would yield 25,000 doses of vaccine, down from his original estimate of 125,000 doses. Expectations shrank further in 1981 when Barry Bloom, in an interview with Nature(1), said that each armadillo, 3 years after infection, would yield 2.5 × 1012 AFB, enough for only 4000 doses. The talisman for bountiful yields had been lost.
By then, 1 had left GSRI to begin the manufacture of leprosy bacilli for WHO at FIT using a fixed IMMLEP protocol based on inoculation of wild-caught armadillos. I had great difficulty in producing tissues containing more than 1010 AFB per g, and was gravely concerned. Low yields were confirmed by a report issued by WHO in 1982(14), stating that the low yield of M. leprae from many armadillos was a cause for concern. They had analyzed the data on sources of inocula, sources of armadillos, length of infection, and bacterial titers of inocula. None of these factors appeared to account for the low yields.
In 1982, WHO had contracts with four laboratories to supply M. leprae . FIT had produced 38% of the usable tissues and, most importantly, 91% of the high-yielding tissues, those containing more than 5 × 109 AFB per g. FIT was comparatively successful but even so our productivity was 2 orders of magnitude lower than WHO had projected at the beginning of the IMMLEP program.
Our final report to IMMLEP illustrates the magnitude of the shortfall. During a period of 14 years, we had produced only 265 animals (out of a total of 637 inoculated) containing more than 109 AFB per g of tissue. Total production was 3 × 1014 M. leprae , about equal to the number found in the liver, spleen, and lepromas of GSRI animal No. 5 (The Table) only 19 months after inoculation. Most importantly, our average yield on the 265 successfully inoculated animals was 1.2 × 1012 AFB per animal compared to 1.7 x 1014 AFB per animal projected by Rees, a 142-fold difference. These figures are unequivocal. The original IMMLEP goals had become impossible to attain.
The WHO Tropical Disease Research Report for 1989-1991 (16) confirmed this catastrophic decline by stating that research on leprosy bacilli was handicapped by the small numbers of bacilli produced by armadillos. The bacilli took 2 years to grow, it said, and yields were limited to 1011 to 1012 M. leprae for each successfully infected animal. Nevertheless, the report continued, despite limitations of growing M. leprae in armadillos, significant progress was made toward understanding the organism.
WHO damned the armadillo with faint praise. As a source of bacilli, it had fallen far short of expectations, yielding less than 1% of the extrapolated number of M. leprae . The problems saddled on vaccine researchers by low AFB titers are lamented in a 1982 WHO report(14): "Purification of the bacteria from infected tissue has been a major subject of the research. The criteria of high recovery, preservation of bacterial antigens, and elimination of host derived material are not easily reconciled. The method currently used achieves high yield. . . . Some problems remain: (i) The suspensions from some batches of liver tissue . . . are contaminated with a particulate iron-containing brown pigment.... (ii) The bacteria are contaminated by absorbed host components . . . (iii) There is strong evidence that limited proteolysis occurs during homogenization and that bacterial polypeptides are degraded. . . . (iv) Several workers attempting the purification process have been concerned about low recoveries . .. attempts to process tissues with very low bacterial counts (109/g) seem to result in poor yields, probably because of ineffective pelleting of the bacteria, (v) The possibility that some of the observed properties of M. leprae are related to its growth in the armadillo host should be considered.... Meanwhile, attempts to cultivate M. leprae in culture should be encouraged."
The clouds of frustration and wishful thinking enveloping vaccine development could have been dispelled in an instant by a bountiful supply of tissues containing >1011 AFB/g. Any metallurgical engineer knows that a smelter designed to process ores containing 10% of the sought-after metal will not function properly if the feedstock drops to 0.1 %. It is not surprising that 2 of 6 lots of vaccine produced for WHO did not meet minimum standards(15) and field trials on 141,000 volunteers in Venezuela(2) and Malawi(4) showed that the vaccine did not afford significant protection against leprosy infection. A trial is still in progress on the IMMLEP vaccine on 37,000 volunteers in South India. However, India has already bypassed it by approving a vaccine based on Mycobacterium w . (s). The IMMLEP program did not result in an effective vaccine and did not even give a clear-cut answer as to whether such a vaccine is possible.
What caused this catastrophe? The answer may be amazingly simple. At GSRI, we harvested most of the tissues supplied to WHO from young animals born and raised in captivity(11). Thus, they were isolated from soil mycobacteria from time of birth which prevented them from acquiring mycobacterial immunity. In Florida, all of the armadillos inoculated for the IMMLEP program were wild-caught adults with acquired cross-immunity. The immunologic gap between the two groups could have caused the difference.
We did not use laboratory-reared animals at GSRI with the specific intent of increasing yield, although we realized this was a distinct possibility(13). At first we used them to achieve our long-range goal of developing an inbred strain of armadillos highly susceptible to leprosy. Immunologic naivete was an automatic result of the overall research plan. Later, we used them in efforts to avoid inoculating wild-caught adults that we feared might be infected with the then unknown organism causing a leprosy-like disease in wild armadillos(12). When the GSRI leprosy program was terminated prematurely, the talisman leading to high productivity was lost.
I cannot prove that the decline of armadillo productivity occurred exclusively because of acquired immunity of wild-caught animals, but the basic concept is enshrined in medical lore. For generations, leprologists have preached that acquired immunity protected white settlers in Hawaii from infection during the storied leprosy outbreak of the 19th century when multibacillary leprosy led to the exile of immunologically naive Polynesians to Molokai. Regardless of the mechanism, I am certain that yield did not dwindle by more than 2 orders of magnitude without cause. This cause must be sought. M. leprae has not yet been grown in artificial media. Armadillos are still the major source of supply.
Premature termination of the GSRI program may have killed the hopes for an antileprosy vaccine irrevocably, but many research goals of the future will require bountiful supplies of M. leprae . These could be obtained by rediscovering the talisman lost at GSRI or pursuing the seemingly endless quest for cultivation in artificial media. Eperience has shown that it is easier to restore a lost art than to create a new one for which there is no pre-existing template. I earnestly hope that the potential of the armadillo will be re-evaluated by the next generation of leprologists. Perhaps the lost talisman can be rediscovered.
- Eleanor E. Storrs, Ph.D.
72 Riverview Terrace
Indialantic, Florida 32903, U.S.A.
REFERENCES
1. Anon. Armadillos light leprosy. Nature 291(1981)527.
2. Convit, J., Sampson, C., Zuniga, M., Smith, P. G., Plata, J.. Silva, J., Molina, J., Pinardi, M. E., Bloom, B. R. and Salgado, A. Immunoprophylactic trial with combined Mycobacterium leprae /BCG vaccine against leprosy: preliminary results. Lancet 339 (1992) 446-450.
3. Draper, P. Leprosy review: the bacteriology of Mycobacterium leprae . Tubercule 64(1983)43-56.
4. Karonga Prevention Trial Group. Randomized control trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348(1996)17-24.
5. Kato, L., Ishaque, M. and Walsh, G. P. Cytochrome pigments in Mycobacterium leprae isolated from armadillos (Dasypus novemcinctus L.). Microbios 12(1975)41-60.
6. Maugh, T. H. Leprosy vaccine trials to begin soon. Science 215(1982)1083-1086.
7. Meyers, W. M. Leprosy research and patient care over the past century. Int. J. Lepr. 66(1998)43-48.
8. Nath, I. A vaccine for leprosy. Nature Medicine 4(1998)548-550.
9. Rees, R.J.W. The WHO program for research on immunology of leprosy (IMMLEP). B. Some areas of scientific progress. Int. J. Lepr. 44(1976)280-283.
10. Stewart-Tull, D. E. S. Leprosy-in pursuit of a vaccine. Vaccine 2(1984)238-248.
11. Storrs, E. E. Leprosy in the nine-banded armadillo. Zeit. Tropenmed. Parasitol. 24(1973)53-65.
12. Walsh, G. P., Storrs, E. E.. Burchfield, H. P., Cottrell, E. H, Vidrine, M. F. and Binford, C. H. Leprosy-like disease occurring naturally in armadillos. J. Reticuloendothel. Soc. 18(1975)347-351.
13. Walsh, G. P., Storrs, E. E., Burchfield, H. P. and Virdrine, M. F. New statistics on the incidence of leprosy in the armadillo. Int. J. Lepr. 42(1974)380-381.
14. World Health Organization. Report of the Sixth Annual Meeting of the Scientific Working Group on the Immunology of Leprosy (IMMLEP). Geneva: World Health Organization, 1982, pp. 8, 11. WHO document TDR/IMMLLP-SWG (6)82.3.
15. World Health Organization. Immunology of Mycobacterial Diseases (MMYC) Steering Committee. Analysis of vaccines prepared from armadillo-derived M. leprae ; results of an inter-laboratory study coordinated by the World Health Organization. Int. J. Lepr. 53(1995)48-55.
16. World Health Organization. Tropical Diseases Research Program. Progress in Research, 1989-1990. Geneva: World Health Organization, 1991, pp. 95-96.