- Volume 67 , Number 1
- Page: 71–5
Effect of Zafirlukast on leprosy reactions
To the Editor:
Reactional states in leprosy patients are a significant cause of morbidity. These states can be divided mainly into erythema nodosum leprosum (ENL) and reversal reaction (RR). Clinically, ENL is characterized by the development of groups of new, small, tender erythematous subcutaneous nodules which usually subside after a few days along with systemic features like pyrexia, arthralgia, neuritis, and adenopathy. RR, on the other hand, presents as brawny, indurated, erythematous skin plaques with acute or chronic neuritis as a prominent feature. Leprosy reactions need to be controlled in a rapid and effective manner in order to prevent progressive nerve damage. The medications to suppress these conditions are thalidomide for ENL and high doses of oral glucocorticoids for both ENL and RR(7-9). Some patients require chronic steroid use, putting them at risk for several adverse side effects. Besides its well-documented teratogenic effects, thalidomide has also been shown to cause neuropathies(11). Due to the high side effect profile of these therapies, there is a need to find better treatments associated with less toxicity.
Knowing that reactional states are the result of a cytokine imbalance of the immune system, different approaches have been made to alter the cytokine milieu. Tumor necrosis factor-αlpha (TNF-α) and interleukin-1 (IL-1) have been implicated as key mediators in Hansen's disease and reactional states (2, l2,l4). Recently, Calhoun, et al . have shown that zafirlukast (AccolateTM) is capable of reducing TNF-α and superoxide anion concentrations in the bronchioalveolar lavage fluid of asthmatic patients stimulated with segmental antigen challenged(3). Their rationale was based on the fact that Chan, et al . showed that monocytic cells express on their surface cysLTDl receptors that are inhibited by zafirlukast(4). We decided to conduct an open phase II cohort trial to test the effect of zafirlukast on leprosy reactional states.
Zafirlukast is a U.S.A. Federal Drug Administration (FDA) approved immunomodulator which together with montelukast and pranlukast belongs to the family of leukotriene receptor antagonists, and has been used for the prophylaxis and treatment of asthma in adults. It is known to be a potent inhibitor of inflammation due to its selective antagonistic effect at the level of the cysteinyl leukotriene receptor. Zafirlukast blocks the final inflammatory products of the arachidonic acid-eicosanoid cascade that are processed through the 5-lipoxygenase pathway(1,5,10). This drug is well tolerated with an incidence of adverse effects similar to that seen with the use of placebo (16). The most common adverse effects reported have been headache and pharyngitis (l7). In addition, the dosing regimen of twice a day orally increases patient compliance.
Patients were selected from the New York Hansen's Disease Program of Staten Island University Hospital, New York, U.S.A., during the period between June 1997 and June 1998. Patients were classified according to the Ridley and Jopling defined states and categories: polar tuberculoid (TT), borderline tuberculoid (BT), borderline undefined (BB), borderline lepromatous (BL), and polar lepromatous (LL)(13). All leprosy patients with reactions were evaluated and eligible for entry into the study. Reactional states were identified and classified according to patient's symptoms and signs. The severity of the reactions ranged from mild to moderate. Clinically, ENL was defined as the appearance of tender, erythematous, subcutaneous nodules with or without systemic manifestations such as fever, arthralgia, and adenopathy. RR was defined as acute or chronic neuropathic pain and usually associated with mixed motor and sensory loss. Informed consent was obtained from patients, and the study was approved by the local Institutional Review Committee.
All patients were given starting doses of oral zafirlukast 40 mg b.i.d. and were kept on the medication as long as they were able to tolerate it or as long as the reactional state was still active. In some patients the dose was reduced to 20 mg b.i.d. after the reaction subsided. If the patients were already taking steroids, their prednisone doses were kept the same but were never increased. The goal was to taper the prednisone doses gradually on steroid-dependent patients. In addition to the above medications, patients were treated with various combinations of dapsone, clofazimine, and rifampin according to standard U.S. guidelines(7-9). During the time of the study only two patients (18 and 23) were taking thalidomide.
One observer (William R. Levis, M.D.) followed the patients throughout the study to evaluate their responses which were divided into five categories, each with a descriptive and numerical value. Definition of responses: Excellent (5) = complete resolution of all symptoms and signs of the reactions: disappearance of ENL lesions and systemic manifestations or complete relief of neuropathic pain in RR and reinstitution of sensation; Good (4) = resolution of most symptoms and signs: flattening of the skin lesions of ENL or incomplete relief of neuropathic pain and incomplete restoration of sensory level in RR; Moderate (3) = resolution of some symptoms and signs: slight improvement in the severity of ENL lesions and slight improvement in the control of neuropathic pain of RR without restoration of sensory level; No Response (2) = no effect of therapy; Worse (1) = worsening of symptoms and signs while on therapy.
RESULTS AND DISCUSSION
A total of 25 patients completed the study. The age of the patients ranged from 18 to 82 years (mean age 43.6; standard deviation of 17.15 years). The sample consisted of 18 males (72%) and 7 females (28%). All of the patients were born outside of the U.S.A. Most of the patients had LL (10), followed by 8 with BT, and 7 with BL. Nineteen patients had RR and 6 had ENL (Table 1). The patients were treated with oral zafirlukast doses of 20 to 40 mg twice a day, depending on their ability to tolerate the side effects. The mean length of time on zafirlukast at the time of the follow up was 3.9 months (95% confidence interval 2.7 to 5.05).
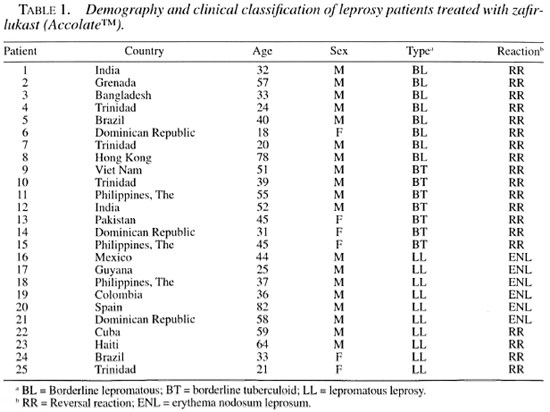
The response rates were excellent in 48% (12/25) of the patients (Table 2). These consisted of 4 LL ENL, 2 LL RR, 3 BT RR, and 3 BL RR. Forty-four percent (11/25) had a good response, and this group consisted of 5 BT RR, 2 LL ENL, 2 LL RR, and 2 BL RR. One patient with BL RR had a moderate response and one had no response to zafirlukast. This last patient only received the drug for 5 days and had to return to steroids. No worsening of the reactions were reported in any of the patients while on treatment. No significant differences were noted between ENL and RR on response or among the different categories. Both females and males responded similarly to treatment. Most of the patients responded with symptomatic improvement after at least 2 weeks of treatment. Remission of symptoms was evident with the disappearance of the erythematous nodules of ENL or with the restoration or improvement of the distal sensory level and disappearance of neuropathic pain. Patient 19 had a resolution of a left proximal ulnar nerve abscess while taking zafirlukast.
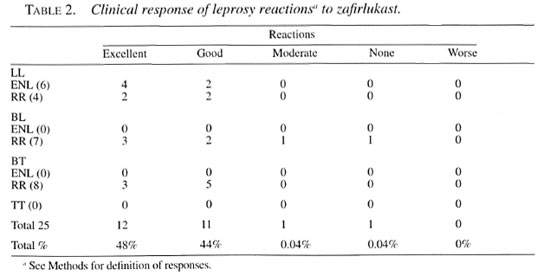
Zafirlukast appears to be an effective form of therapy for both ENL and RR in Hansen's disease patients regardless of sex, age or type of reaction. Our results were that 92% of the patients showed an excellent or good response to therapy. The exact mechanism of action of zafirlukast in leprosy reactions is currently unknown. TNF-α is released during leprosy reactions and this, in turn, induces the synthesis of eicosanoids(14). The local effect of eicosanoids (leukotrienes) during skin inflammation has been demonstrated(6,15). Thus, we postulate that a probable mechanism of action is the inhibition of the final products of inflammation, leukotrienes, in the skin. Further studies at the molecular level are needed. In asthma, a disease of Th2 predominance, the role of leukotriene inhibition has been clearly demonstrated to be effective(l6). Zafirlukast may act as an immunomodulator by inhibiting leukotriene receptors in mononuclear cells and thereby decreasing TNF-α production as has been shown in asthmatic patients(3,4).
Thirteen of the 25 patients had chronic RR which were difficult to control and had become chronically dependent on prednisone (52%). Ten of these patients (77%) were able to significantly reduce their steroid doses, to the extent that 4 of 13 (30%) were able to discontinue prednisone completely. Only one patient needed an increased dose of steroids to control the RR; this was the same patient who took zafirlukast for 5 days only. Even more important was that 12 patients (48%) did not need steroids to control their reactions. As the steroid doses were decreased, none of the patients experienced recurrence of reactions and, therefore, none were restarted on steroids. However, when the zafirlukast was decreased from 40 to 20 mg b.i.d., either the neuropathic pain or the ENL recurred. These symptoms were controlled within 2 weeks by returning to the previous dosage, thus exhibiting a dose-related response. None of the patients in whom prednisone was decreased presented symptoms or signs of Churg-Strauss allergic vasculitis which has been reported in some asthmatic patients during corticosteroid withdrawal(18).
As stated previously, only two patients had been taking thalidomide. However, their reactions were suppressed only after starting zafirlukast, perhaps meaning that leukotriene inhibition played a key role. The only adverse effect described was headache, which resulted in three patients discontinuing the drug. One of them was able to restart the medication after 4 months without re-experiencing headache.
We observed altered liver enzymes in one patient. The aspartate and alanine aminotransferases (AST and ALT, respectively) were modestly increased by less than two times the baseline value (AST 36 U/L, ALT 63 U/L). Patient 11 had a modest elevation of liver enzymes prior to starting zafirlukast. These modest elevations of liver enzymes persisted but did not increase during treatment with zafirlukast.
- Eduardo A. Vides, M.D.
Aloys Cabrera, R.N.
Kathleen P. Ahern, Ph.D., RN.
William R. Levis, M.D., Director
Department of Internal Medicine and Dermatology
New York Regional Hansen's Disease Program
Staten Island University Hospital
475 Seaview Avenue
Staten Island, New York 10305, U.S.A.
REFERENCES
1. Adkins, J. C. and Brodgen, R. N. Zafirlukast; a review of its pharmacology and therapeutic potential in the management of asthma. Drugs 55(1998)121-144.
2. Barnes, P. F., Chatterjee, D., Brennan, P. J., Rea, T. H. and Modlin, R. L. Tumor necrosis factor-α production in patients with leprosy. Infect. Immunol. 60(1992)1441-1446.
3. Calhoun, W. J., Lavins, B. J., Minkwitz, M. C., Evans, R., Gleich, G. J. and Cohn, J. Effect of zafirlukast (AccolateTM) on cellular mediators of inflammation: bronchioalveolar lavage fluid findings after segmental antigen challenge. Am. J. Respir. Crit. Care Med. 157(1998)1381-1389.
4. Chan, C. C., Ecclestone, P., Nicholson, D. W., Metters, K. M., Pon, D. J. and Rodger, I. A. Leukotriene D4-induced increases in cytosolic calcium in THP-1 cells; dependence on extracellular calcium and inhibition with selective leukotriene D4 receptor antagonists. J. Pharmacol. Exp. Ther. 269(1994)891-896.
5. Drazen, J. M. Pharmacology of leukotrienes receptor antagonists and 5-lipoxygenase inhibitors in the management of asthma. Pharmacotherapy 17 (1997)22-30.
6. Fretland, D. J., Gokhale, R., Mathur, L., Baron, D. A., Paulson, S. K. and Stolzenbach, J. Dermal inflammation in primates, mice, and guinea pigs: attenuated by second-generation leukotriene B4 receptor antagonist, SC53228. Inflammation 19(1995)333-346.
7. Jacobson, R. R. Treatment of leprosy. In: Leprosy. 2nd ed. Hastings, R. C., ed. Edinburgh: Churchill Livingstone, 1994, pp. 317-349.
8. Jacobson, R. R. The treatment of leprosy (Hansen's disease). Hosp. Form. 17(1982)1076-1091.
9. Levis, W. R. Treatment of leprosy in the United States. Bull. N. Y. Acad. Med. 60(1984)696-711.
10. O'Byrne, P. M. Leukotrienes in the pathogenesis of asthma. Chest 111(1997)27-34.
11. Ochonisky, S., Verroust, J., Bastuji-Garin, S., Gherardi, R. and Revuz, J. Thalidomide neuropathy incidence and clinicoelectrophysiologic findings in 42 patients. Arch. Dermatol. 130(1994)66-69.
12. Parida, S. K., Grau, G. E., Zaheer, S. A. and Mukherjee, R. Serum tumor necrosis factor-α and interleukin 1 in leprosy and during lepra reactions. Clin. Immunol. Immunopathol. 63(1992)23-27.
13. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
14. Sarno E. N., Grau, G. E., Vieira, L. M. and Nery, J.A. Serum levels of tumor necrosis factor-α and interleukin-1β during leprosy reactional states. Clin. Exp. Immunol. 84(1991)103-108.
15. Soter, N. A., Lewis, R. A., Corey, E. J. and Austern, K. F. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J. Invest. Derm. 80(1983)115-119.
16. Spector, S. L. Management of asthma with zafirlukast; clinical experience and tolerability profile. Drugs 52(1996)36-46.
17. Spector, S. L., Smith, L. J., Glass, M. and the AccolateTM Asthma Trialist Group. Effects of 6 weeks of therapy with oral doses of ICI 204. 219, a leukotriene D4 receptor antagonist, in subjects with bronchial asthma. Am. J. Respir. Crit. Care Med. 150(1994)618-623.
18. Wechsler, M. E., Garpestad, E., Flier, S. R., Kocher, O., Weiland, D. A., Polito, A. J., Klinek, M. M., Bigby, T. D., Wong, G. A., Helmers, R. A. and Drazen, J. M. Pulmonary infiltrates, eosinophilia, and cardiomyopathy following corticosteroid withdrawal in patients with asthma receiving zafirlukast. JAMA 279(1998)455-157.
Reprint requests to William R. Levis, M.D., Director, New York Regional Hansen's Disease Program, Staten Island University, 475 Seaview Avenue, Staten Island, NY 10305, U.S.A.