- Volume 67 , Number 2
- Page: 162–4
Infection by M. leprae is governed by the temperature at the entry point: a preliminary note
This department is for the publication of informal communications that are of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this Journal is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the Journal and thus interfere with its prime purpose.
To the Editor:
The portal of entry of Mycobacterium leprae , the mode of transmission of leprosy in the community, and the evolution of the disease in an individual is still not clear. Previous studies conducted in nude mice have shown that M. leprae can be transmitted through unbroken nasal mucosa (1, 3) and abraded skin (2). It is well known that the nasal mucosa and feet are relatively cooler sites of the body. Infecting the nude mouse through intact and abraded skin at the flank was not successful (3). In this preliminary experiment an attempt is made to find out whether infection can take place if organisms are injected into the flanks of T900r mice which are almost similar to nude mice in their immunological status.
A group of 10 CBA mice were made im- munodeficient using standard methodology except that the bone marrow transplantation was performed by injecting the harvested bone marrow into the peritoneal cavity (4). The M. leprae suspensions were obtained from harvesting the lepromatous nodules from the foot pads of a T900r mouse inoculated 15 months earlier with M. leprae . M. leprae suspensions were diluted so that 0.1 ml of a sample contained 107 M. leprae . In 10 T900r mice, hair was removed from both flanks and 0.1 ml of a suspension containing 107 bacilli was injected intradermally on both sides. Also, 0.03 ml of the bacillary suspension containing 104 bacilli was injected into the hind foot pads of five normal CBA mice to confirm the viability of the bacilli. The animals were fed with standard food pellets and unlimited water. At 6, 12, and 15 months one animal from each group and two animals at 18 and 21 months were sacrificed. Three animals died due to unknown causes. The skin along with the subcutaneous tissue and muscle at the injected area on both sides were dissected out. The tissue from the left flanks was harvested and the acid-fast bacilli (AFB) counted. The tissue from the right flanks was immediately fixed in 10% buffered formalin and used for histopatho- logical studies. The animals were autopsied and the organs were studied grossly and histopathologically. The tissues obtained at autopsy were also fixed in 10% buffered formalin and processed for 4-pm-thick paraffin sections, and stained with hematoxylin and eosin (H&E) and a modified Fite-Faraco stain.
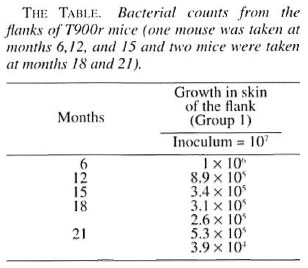
At 6, 9, 12, 18, and 21 months' growth up to 105 AFB was obtained from the foot pads of normal mice, confirming that the M. leprae used in this experiment were viable. The results of the bacterial count obtained from the T900r animals are given in The Table, showing a steady decrease in the number of organisms inoculated into the flanks.
Sections of skin from the flanks showed a diffuse granuloma occupying the dermis and the subcutaneous tissue including bundles of striated muscles. The granuloma consisted of macrophages, some with granular and some with vacuolated cytoplasm, lymphocytes, plasma cells and a few polymorphs (Fig. 1). They were mostly arranged in scattered clumps. Acid-fast staining showed macrophages filled with AFB. Almost all AFB were fragmented and granular (Fig. 2). AFB were not found in any other areas. The above changes were seen consistently at 6 and 15 months. No histopathological changes were observed in the biopsy specimens obtained at month 12, 15 or 21. The hind foot, ears, liver, spleen, lungs, heart and kidneys obtained at autopsy from animals inoculated at the flanks showed no significant lesion except for a small, focal, nonspecific, mononuclear cell inflammatory infiltration of the liver and lungs. No acid-fast organisms were seen.
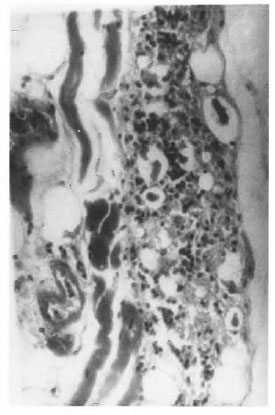
Fig.1. Photomicrograph showing microgranuloma in the skin of the flank of T900r mice 6 months after inoculation with 107 viable M leprae (H&E x250).
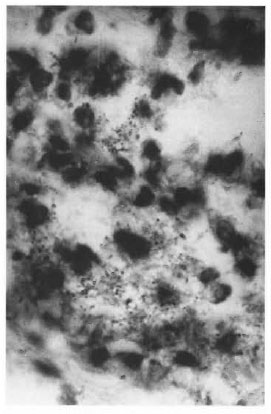
Fig . 2. Photomicrograph showing clumps of broken and granular AFB in macrophages of the granuloura hown in Figure 1 (modified Fite x800).
This study shows that when M. leprae were injected intradermally into the skin of the flanks of T900r mice they were taken up by the macrophages which aggregated in the injected sites. Clumps of macrophages containing AFB were seen at the injected site at 6 and 15 months. Morphologically, the AFB were all fragmented and granular. Their counts decreased from 107 to 106 at 6 months, 8.9 x 105 at 12 months, 3.4 x 105 at 15 months, 3.1 x 105 at 18 months and 3.9 x 104 at 21 months, clearly showing that there was no multiplication at the injected sites. There was also no evidence of dissemination of the infection to other parts of the body since autopsy of the animals did not show any organisms in organs such as the lungs, liver, spleen or ears.
One possible reason for the lack of multiplication may be that the skin at the flanks is warmer than that at the foot pads. It is reasonable, therefore, to suggest that although M. leprae may enter into the body through any skin site it can multiply and disseminate only in a suitable environment with a temperature less than normal core body temperature. The other reason may be that unlike the foot pad, the region of the flanks is a large area and the injected M. leprae may have fanned out without being localized and are thus lost to further evaluation.
The absence of granuloma or any nisto- pathological changes at the right flanks of the animal examined at intervals of 12, 15, and 21 months, although AFB were harvested from the left side, is difficult to explain. The fact that even minimal inflammatory changes were absent suggests that the biopsy might have missed the site completely.
Further experiments are being done to confirm this study since the number of mice used here was small, and there were no control animals infected in the foot pads using the same inoculum and the same dosage for comparison.
- Gigi J. Ebenezer, M.D.
Head, Department of Histopathology and Experimental Pathology
- Shantha Arumugam
Laboratory Technician
Department of Experimental Pathology
- Charles K. Job, M.D., F.R.C.Path.,
F.A.M.S.
Emeritus Scientist
Schieffelin Leprosy Research and Training Center
Karigiri, Vellore District
Tamil Nadu, India 632 106
REFERENCES
1. Chehl, S., Job, C. K. and Hastings, R. C. Transmission of leprosy in nude mice. Am. J. Trop. Med. Hyg. 34(1985)1161-1166.
2. Job, C. K,. Chehl, S. and Hastings, R. C. New finding on the mode of entry of M. leprae in nude mice. (Letter) Int. J. Lepr. 58(1990)726-729.
3. McDermott-Lancaster, R. D. and McDougall, A. C. Mode of transmission of M. leprae infection in nude mice. Int. J. Exp. Pathol. 71(1990)689-700
4. Shantha, A., Job, C. K. and Ebenezer, G. J. Preparation of thymectomized and irradiated T900r mice: a modification. Indian J. Lepr. 69(1997)200-201.