- Volume 67 , Number 2
- Page: 171–2
Detection of antibodies toward secreted mycobacterial antigen 85 in untreated leprosy patients' sera
To the Editor:
Leprosy is an insidious disease that affects two million persons worldwide (1993) and continues to present a public health problem in various parts of the world. The efforts carried out by the World Health Organization (WHO) to eliminate leprosy by the year 2000 have been based mainly on monitored multidrug treatment (3), which includes the use of new diagnostic, prevention and disease classification methods.
Mycobacterium leprae is one of the first human pathogens to have been described, but the impossibility of its cultivation in vitro has impeded the isolation and characterization of its various antigenic components. It would be extremely important to determine the role these antigens may play in the immunopathology of the disease, both in humoral and cellular responses.
The chemical structure of M. leprae is complex. Considering the high degree of homology between this bacterium and M. bovis (1), in this study we analyze the humoral response to M. bovis secreted antigens in patients with Hansen's disease and in normal persons who are contacts of leprosy patients. We used mycobacterial proteins, among them complex 85, actively secreted by this mycobacterium in culture media (4).
We studied sera from 17 multibacillary (LL, BL, BB) patients, 15 paucibacillary (TT, BT, LI) patients, and 10 Mitsuda-positive contacts and nonreiated persons, all adults, to detect antibodies toward M. bovis secreted and M. leprae cytoplasmic proteins by an immunoenzymatic assay.
The antigen used was M. bovis excreted protein. Danish BCG strain 1331 was cultured in Sauton medium (7). Ten freeze-dried BCG ampules (0.5 mg each) were diluted in 200 ml Sauton and seeded in Roux vials for a total final volume of four liters of medium, which was incubated at 37°C for 6 weeks. The medium was then filtered through Whatman No. 1 filter paper, cen- trifuged at 10,000 rpm (Sorvall RC-5B, rotor SS-34) at 4°C for 15 min and finally filtered through a 0.45-µm membrane. Ammonium sulfate (85% saturated) was added to each liter of medium with gentle shaking for 30 min. After centrifugation, the precipitate was resuspended in 100 mM TRIS- HCL, pH 7.4, and dialyzed for 48 hr against the same buffer. The material was concentrated using l0x polyethylene glycol. Protein concentration was determined by the BCA method (8).
The immunoenzymatic assay was performed as described previously (5). Circulating IgG values against excreted proteins were expressed in optical density (OD). Briefly, microtiter plates were sensitized with 2 µg protein/ml, 50 µl per well. All test sera were used at a 1:200 dilution. The secondary serum (peroxidase-labeled antihuman IgG) was used at a 1:5000 dilution.
The substrate was 0PD-H202 and the reaction was stopped with 2.5 NH2S04. Readings were done at 490 nm.
For immunoblotting, the BCG excreted proteins were separated by electrophoresis in polyacrylamide gels containing 10% SDS (6). Samples (500 µg protein) were diluted in sample buffer pH 6.8 (62 mM TRIS-HCL, 2% SDS, 50 mM 2-mercap- toethanol and 10% glycerol), boiled for 3 min and applied to 11-cm gels. Running conditions were 25 mA (constant current) in the stacking gel and 35 mA in the separation gel until bromphenol blue reached the lowest part of the gel. The gel was stained with 0.25% Coomassie brilliant blue. A Sigma standard molecular weight mixture was used.
Proteins in the preparative gel were electrophoretically transferred to 0.45-µm nitrocellulose paper using a pH 8.3 buffer (25 mM TRIS base, 192 mM glycine and 20% methanol) at a constant current of 100 mA for 16 hr (9). Nitrocellulose membranes were cut into 4-mm thick strips and confronted with patient and control sera as follows: 2 hr incubation with test sera 1:400), three 10-min washes with PBS-Tween, 2 hr incubation with secondary peroxidase-labeled anti-IgG serum (1:5000), and three washes as above. 1-4 Chloro-naphthol-H202 was added and the reaction was stopped with water.
The results showed greater intensity of the IgG antibody response directed toward a protein complex in the excreted antigens (The Table), with a relative mobility of approximately 30 kDa, in multibacillary as compared to paucibacillary patients (The Figure).

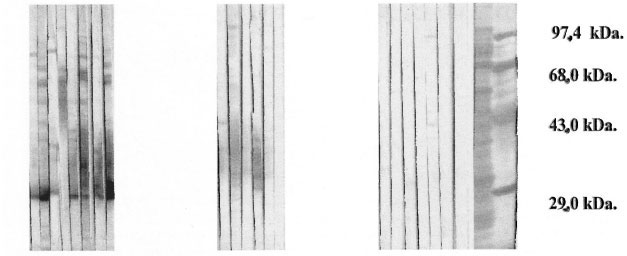
The figure. Immunoblotting of BCG excreted proteins in the presence of sera from Hansen's disease patients.
Other investigators have found low levels of antibodies directed toward secreted proteins (28.5% in multibacillary and 32.3% in paucibacillary untreated patients) (2), and they suggest that there might be immune complexes associated to free antigen. This study does not correlate with the bacterial index or ELISA positivity.
Since M. bovis excreted proteins possess high homology with M. leprae , these results could be measuring bacterial viability; they suggest that these proteins could be candidates for serological follow up of multidrug therapy in leprosy.
- Elsa Rada-Schlaefli, M.Sc.
Carlos Santaella, M.Sc.
Nacarid Aranzazu, M.D.
Jacinto Convit, M.D.
Institute de Biomedicina
Apartado 4043
Caracas 1010A, Venezuela
REFERENCES
1. Harboe, M. and Wiker, H. G. Secreted proteins of Mycobacterium leprae . Scand. J. Immunol. 48(1998)577-584.
2. Mistry, N. F., Iyer, A., Harboe, M. and Antia, N. H. Low rates of detection of mycobacterial secretory antigen 85 in sera of untreated leprosy patients. Int. J. Lepr. 64(1996) 451-453.
3. Noordeen, S. K. Elimination of leprosy as a public health problem and prospects. Bull. WHO 73(1995) 1-6.
4. Pesolani, M. C. V. and Brennan, P. J. Mycobacterium leprae produced extracellular homologs of the antigen 85 complex. Infect. Immun. 60(1992)4452-4459.
5. Rada, E., Aranzazu, N. and Convit, J. Immunological reactions to mycobacterial proteins in the spectrum of leprosy. Int. J. Lepr. 65(1997)497-500.
6. Rada, E., Santaella, C., Aranzazu, N. and Convit, J. Preliminary study of cellular immunity to M. leprae protein in contacts and leprosy patients. Int. J. Lepr. 60(1992) 189-194.
7. Sadamu, N., Nagasuga, T., Matsumoto, J. and Kohda, K. Isolation of tuberculin skin reactive proteins from heated culture filtrate of Mycobacterium tuberculosis H37Rv. Am. Rev. Res. Dis. 109(1974) 17-27.
8. Smith, P. K. Measurement of protein using bicin-chonic acid. Anal. Biochem. 150(1985)76-85.
9. Towbin, H., Staehei.in, T. and Gordon, J. Electrophoretic transfer of proteins from Polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc. Natl. Acad. Sei. U.S.A. 76(1979)4350-4354.