- Volume 67 , Number 2
- Page: 159–61
Efficacy of WHO regimens in the management of leprosy patients with G6PD deficiency
In an effort to increase the utility of the Journal in continuing medical education, in this section we welcome contributions dealing with practical problems in leprosy work. Submissions to this section will undergo minimal editorial changes and may well contain controversial points. Letters to the Editor pointing out oother viewpoints are welcome.
The World Health Organization (WHO) regimens of multidrug therapy (MDT) for the treatment of leprosy have proved to be highly safe and efficacious, resulting in a remarkable reduction in the worldwide prevalence of leprosy1. Dapsone, along with rifampin (RMP) and clofazamine (CFZ) are the three drugs used in these regimens.
In Oman, leprosy has been eliminated as a public health problem (prevalence rate 0.18/10,000); 17 new cases were detected in the year 1996. The number of cumulative cases registered and treated between 1991-1996 was 166. An important feature of the leprosy situation in Oman is the high prevalence of G6PD deficiency in the local population. It has been reported that the prevalence of the deficiency may be as high as 21% in males and 11 % of homozygous females-the second highest in any population2. The most common prevalent variants of the enzyme are G6PD Mediterranean and G6PD A-. These enzyme variants have reduced functional activity, and often are associated with oxidative hemolysis in the presence of stress factors such as drugs, ingestion of fava beans and infections.3
Dapsone, an extremely safe, cheap and effective drug, is known to cause oxidative hemolysis in patients with G6PD deficiency.3-4 Caution has to be exercised in the use of the drug in such individuals. The hemolysis caused by dapsone in these patients has been reported to be variable and depends on a number of factors, such as the type of enzyme variant, dose of drug used, and the acetylator status of the patient.5-9 This has led to differences in opinion about whether the drug should be omitted in all patients with G6PD deficiency.3-7 WHO has recommended that the drug needs to be discontinued in patients developing severe hemolysis, and alternative drugs instituted as follows:1 Paucibacillary (PB) leprosy = RMP 600 mg once monthly + CFZ 300 mg monthly with 100 mg on alternate days for 6 months. Multibacillary (MB) leprosy = RMP 600 mg once monthly + CFZ 300 mg monthly with 100 mg on alternate days for 24 months.
In view of the high prevalence of the deficiency in Oman and the risk of the occurrence of hemolysis, our current policy, as advocated by the Ministry of Health, Sultanate of Oman, is to screen all leprosy patients for the deficiency and to avoid dapsone in all patients with the deficiency. These patients are treated with the alternative drug regimens mentioned above. We hereby report our experience regarding the efficacy of these alternative regimens in these patients.
METHODS AND RESULTS
Patients registered and treated during the years 1991-1996 were studied with respect to their G6PD status. Eighteen patients were found to have G6PD deficiency, amounting to 10.84% of all the patients. Table 1 shows the details of these patients. As can be seen, the male : female ratio was 2:1. There were 14 MB leprosy patients (including 10 LL, 3 BL, and 1 BB) and 4 PB leprosy patients (2 TT and 2 BT). The initial bacterial index (BI) and the response to treatment of these patients are shown in Table 1.
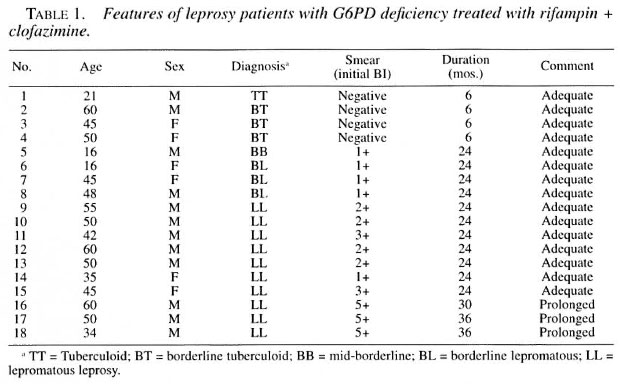
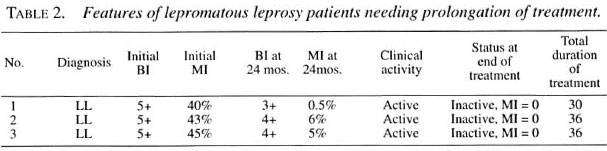
All PB patients responded well to treatment and the treatment was stopped at 6 months. Among the MB patients, 11 cases became clinically inactive and smear negative at the end of 24 months. In three cases with lepromatous leprosy, who had a high initial BI of 5+, a review of the patients at the end of 24 months showed clinical signs of activity (Table 2), such as persistent infiltration of earlobes, nodules and plaques over the face and trunk. Morphological indexes (MI) showed persistent bacteriological activity. In these three patients, the treatment was continued beyond the recommended 24 months, until clinical and bacteriological inactivity was reached. Lepra reaction was not noted in any patient, and the drugs were well tolerated.
DISCUSSION
G6PD deficiency was seen in 10.84% of the patients, consistent with the high rates mentioned above. Our study shows that the alternative regimens recommended by WHO in G6PD-deficient patients are effective, and that avoidance of dapsone does not seem to alter the prognosis of these patients.
Recent WHO guidelines1,10 recommend the use of newer antileprosy drugs, such as minocycline, ofloxacin, and clarithromycin, as alternatives for the above standard drugs in case of drug resistance and intolerance to rifampin and clofazimine. However, these WHO guidelines do not recommend the use of any of these newer drugs for patients with G6PD deficiency.1-10 It is interesting to note that in three florid LL cases with a high initial BI in our study, treatment had to be continued beyond the recommended 24 months. These patients had received only two drugs (rifampin and clofazimine) as per the current WHO guidelines.1
However, the number of patients in our study is small, and even with the standard MDT with a three-drug regimen, LL cases with a high BI have been reported to show persistent clinical activity.1 Hence, it is not possible to draw definite conclusions about the lack of efficacy of the two-drug regimen used in these cases. However, we feel that this subject of management of patients with G6PD deficiency with two drugs needs careful scrutiny by proper controlled trials. Further, in view of the high prevalence of the deficiency in several parts of the world, studies should be carried out to determine the efficacy of alternate regimens, such as the ROM regimen (monthly administration of 600 mg of rifampin, 400 mg of ofloxacin, and 100 mg of minocycline).
- Venkataram Mysore, M.D.
Junior Specialist
- Abdul Raouf Al-Suwaid, M.D.
Senior Consultant and Chief
Department of Dermatology and Genito Urinary Medicine
Al Nahdha Hospital and Baushar Polyclinic
Muscat, Sultanate of Oman
1. Who Study Group. Treatment of Leprosy . Geneva: World Health Organization, 1993, pp. 1-5. Tech. Rep. Ser.
2. White, J. M., Chrisie, B. S., Nam, D., Daar, S.and Higgs, D. R. Frequency and clinica) significanceof erythrocyte genetic abnormalities in Omanis. J.Med. Genet. 30(1993)396-400.
3. Beutler. E. Glucose 6 phosphate dehydrogenase deficiency-a revicw article. N. Engl. J. Med. 324(1991) 160-174.
4. Cox, A. and Roberts, J. Dapsone in G6PD deficient individuais. (Letter) N. Engl. J. Med. 324(1991)1742-1743.
5. Beutler, E. Reply to the editor. N. Engl. J. Med. 324(1991)1743.
6. DeGowin, R. L. A review of therapeutic and hemolytic effects of dapsone. Arch. Intern. Med. 120(1967)242-248.
7. Degowin. R. L.,Eppes, R. B.,Powel, R. D. and Caron, P. E. The hemolytic effects of diphenylsul-phone in normal subjects and in those with glucose 6 phosphate dehydrogenase deficiency. Bull. WHO 36(1966) 165-179.
8. Mgon, A. M., Leipzig, R. M., Zannoni, V. G. and Brewer, D. J. Interaction of glucose 6 phosphate 6 dehydrogasc deficiency with drug acetylation and hydroxylation. J. Lab. Clin. Med. 97(1981)764-770.
9. Byrd, S. R. and Gelber, R. H. Effect of dapsoneon hemoglobin conecntration in patients with leprosy. Lepr. Rev. 62(1991)171-178.
10. Who Action Programme for the Elimination of Leprosy. MDT- Questions and Answers . rev. edn.Geneva: World Health Organization, 1997, p. 20.
Reprint requests to Dr. Venkataram Mysore, Consultant Dermatologist, Post Box 12, Salmaniya Medical Complex, Manama, State of Bahrain.