- Volume 67 , Number 3
- Page: 250–8
Disabilities in multibacillary leprosy following multidrug therapy with and without immunotherapy with Mycobacterium w antileprosy vaccine
ABSTRACT
A vaccine based on autoclaved Mycobacterium w was administered, in addition to standard multidrug therapy (MDT), to 157 bacteriologically positive, leprominnegative, multibacillary leprosy patients supported by a well-matched control group of 147 patients with similar type of disease who received a placebo injection in addition to MDT The MDT was given for a minimum period of 2 years and continued until skin-smear negativity, while the vaccine/placebo was given at 3-month intervals up to a maximum of 8 doses in the initial 2 years. The overall incidence of type 1 and type 2 reactions and neuritis during treatment and follow up was nearly equal in the patients in the vaccine and placebo groups; the differences were not statistically significant. The occurrence of disabilities, such as anesthesia, trophic ulcers, claw hand and grade 3 deformities, were not different statistically in the vaccine and placebo groups, an observation valid both for deformities present at induction and for those which developed during the course of therapy and surveillance. A statistically significant difference was observed in the recovery of newly developed trophic ulcers; recovery was quicker in the vaccine group. The recovery rate for motor deformities was marginally higher in the vaccine group, although not significant (p = 0.068) statistically. There was a statistically significant reduction in the incidence of grade 3 deformities following MDT with and without immunotherapy. To conclude, the addition of vaccine to MDT did not precipitate neuritis or deformities over and above that encountered with MDT alone, although it did accelerate bacteriological clearance, histopathological upgrading, conversion to lepromin positivity, and clinical improvement.RÉSUMÉ
Un vaccin, préparé à partir de Mycobacterium w. autoclavées, fut administré en plus de la poly-chimiothérapie (PCT) standard à un groupe de 157 patients hanséniens multibacillaires (LL, BL et BB), positifis à l'examen bacterioscopique et négatifs au test de la lépromine. Les résultats furent comparés à un groupe contrôle de 147 patients présentant des types similaires de lèpres, ayant reçu une injection placebo en plus de la PCT. La PCT fut adminstrée pendant une période minimale de 2 années et prologée jusqu'à ce que l'examen de suc dermique fût négatif, tandis que le vaccin fut adminstré touson les 3 mois, avec un maximum de 8 doses pendant les premières années. L'incidence des réactions de type 1, de type 2 et les névrites se sont déclarées durant le traitement et le suivis post traitement fut à peur près identique entre le groupe des patients vaccinés et le groupe placebo de patients; les différences n'étaient pas statisquement significatives. Les complications invalidantes, comme les pertes de sensibilité, les ulcères trophiques, les mains en griffe et les déformations de grade 3 n'étaient pas signficativement différentes entre le groupe vacciné et le groupe contrôle, une observation valable à la fois pour les déformations présentes à l'initiation et pour celles qui se sont développées durant et après le traitement, et au cours du suivi. Une difféference statistiquement significative fut observée en ce qui concerne la vitesse de cicatrisation des ulcères trophiques nouvellement apparus: la récupération fut plus rapide dans le groupe des vaccinés. Le taux de récupération des déformations d'origine motrice fut légèrement supérieur, mais de façon marginale et non statistiquement significative (p = 0,068). Une réduction statistiquement significative de l'incidence des déformations de grade 3 après le début de l'administrion de la PCT, avec ou sans immunothérpae, a été norée. En conclusion, l'addition d'un vaccin au traitement standard PCT n'a pas entraîné plus de névrites ou de déformations plus graves que celles déjà associées à la PCT seule, mais a permis d'accélérer la clairance bactériologique, l'amélioration du grade histopathologique, l'évolution vers un test positif à la lépromine et l'amélioration clinique.RESUMEN
Ciento cincuenta y siete pacientes con lepra multibacilar y lepromino-negativos, se trataron con una vacuna autoclaveada preparada con Mycobacterium w y con la poliquimioterapia (PQT) estándar. Los resultados se compararon con los obtenidos en un grupo de 147 pacientes tratados con placebo (en lugar de la vacuna) y PQT. La PQT se administro durante un período mínimo de 2 anos y se continuo hasta la negatividad de los extendidos de linfa cutânea mientras que la vacuna se administro a intervalos de 3 meses, con un máximo de 8 dosis, durante los 2 anos iniciales de tratamiento. La incidência global de reacciones tipo 1 y tipo 2 y de neuritis, durante el tratamiento y seguimiento, fue casi igual en los dos grupos de pacientes, las diferencias no fueron estadisticamente significativas. Tampoco hubo diferencia en la frecuencia de alteraciones (anestesia, ulceras tróficas, mano en garra y deformaciones grado 3) entre los grupos vacunado y no vacunado, y esto fue cierto tanto para las deformidades presentes al inicio dei estúdio como para las desarrolladas durante el tratamiento y seguimiento. Se observo, sin embargo, una diferencia estadisticamente significativa en la recuperación de las úlceras tróficas de reciente desarrollo; la recuperación fue más rápida en el grupo vacunado. La tasa de recuperación de las deformidades motoras fue marginalmente más alta en el grupo vacunado, aunque la diferencia no fue estadisticamente significativa (p = 0.068). Hubo una reducción estadisticamente significativa en la incidência de deformaciones grado 3 como consecuencia de la PQT con y sin inmunoterapia. En conclusion, la adicion de la vacuna a la PQT no precipito ni neuritis ni deformaciones con más frecuencia que la observada en los pacientes tratados sólo con PQT, aunque si acelero la depuración de bacilos, la mejoria histopatológica, la conversion a la positividad a la lepromina, y la mejoria clínica.Leprosy, a disease known more for morbidity than mortality, causes disability through damage to the peripheral nerves. The worldwide disability rates (for grade 2 and grade 3 disabilities, grade 1 excluded) among leprosy patients vary from 16%50% (3). The reported disability rates in India vary from 16%-44% (7,10). Early diagnosis, regular treatment and early recognition of nerve function impairment are of utmost importance to prevent and reverse nerve damage (5). Several studies have noticed a steady fall in the deformity rate among new cases following the introduction of multidrug therapy (MDT) during the 1980s (1). In our own study of 151 multibacillary (MB) patients, each with a high bacterial index (BI) and a lepromin-negative status and treated with standard MDT until the point of skin-smear negativity, the disability rates varied from 27%-29% for different types of deformities. The occurrence of grade 2 deformities (claw hand, trophic ulcers, etc.) was not found to be affected by MDT because its incidence was nearly equal before MDT and during MDT.
There was a statistically significant difference observed in the incidence of grade 3 deformities (permanent resorption of fingers and toes, including saddle nose) before and after MDT (11.9% against 1.3%) (8). The impact of MDT on the incidence of disabilities and deformities has been reported in a number of studies. However, very limited information is available on the impact of immunotherapy on disabilities. The vaccine based on Mycobacterium w bacilli has shown its ability to accelerate BI clearance, rapid clinical improvement, significant conversion to lepromin positivity and histopathological upgrading in patients receiving vaccine in conjunction with MDT in comparison to those receiving MDT with placebo (14). However, with the use of an immunomodulator in leprosy it becomes necessary to assess its impact on the incidence of neuritis, reactions and deformities since alterations in cell-mediated immunity (CMI) associated with immunotherapy may be one of the precipitating factors for nerve damage. Hence, in this communication an account of the incidence and course of disabilities following the addition of immunotherapy to standard MDT and MDT with a placebo is reported.
MATERIALS AND METHODS
A vaccine based on Mycobacterium w bacilli has been under evaluation for its immunotherapeutic effects as an adjunct to MDT. Our patients were selected from those registered at the Urban Leprosy Centres of Safdarjung Hospital and Dr. Ram Manohar Lohia Hospital in New Delhi, India, during 1987-1992 (12). At these centers, most of the patients are referred by self or by peripheral health centers. During this period, the vaccine was administered along with MDT to untreated, lepromin-negative, multibacillary (MB) leprosy cases, predominantly with a high pre-treatment BI, supported by a well-matched group of patients who received a placebo injection in place of the vaccine along with MDT. The vaccine and the placebo vials were coded since it was a double-blind study; details of the blinding procedure are reported elsewhere in this issue OInjections from alternate vials were given to the patients coming in succession to make sure than an almost equal number of patients fell into each group. The vaccine/placebo administration was continued until a maximum of eight doses were given over 2 years, after which MDT was continued until skin-smear negativity. The patients were followed up after completion of therapy for a period of 5 years. There were 304 (157 vaccine and 147 placebo) patients belonging to the LL, BL and BB clinical types of leprosy (clinical spectrum confirmed histopathologically) included in this study who have been analyzed with reference to their disability grading before, during and after 2 years of therapy.
At induction, all cases were subjected to a detailed clinical examination of the eyes, face, hands and feet, focusing attention on sensory loss, muscle weakness, palmar or plantar ulcers, claw hands or toes, wrist or foot drop, contractures and absorption of fingers and toes. Peripheral nerves were examined for thickening and tenderness. Superficial sensations (temperature, pain and touch) were tested using a temperature tester (supplied by WHO) and pin and cotton wisp, respectively. Voluntary muscle testing was used for assessing the motor functions (4). A pro forma sheet for leprosy reaction was filled in during each reactional episode. Disabilities were classified according to the World Health Organization (WHO) classification (13).
Improvement or deterioration of disabilities were evaluated in the initial and subsequent monthly clinical evaluations. The patients were educated and advised to report immediately if any signs or symptoms of leprosy reaction, neuritis or any other impairments were noted or felt by them. Mild reactions (both type 1 and type 2) were managed with rest and nonsteroidal antiinflammatory drugs (NSAID). Cases with severe reactions (both type 1 and type 2) and neuritis were treated with initial hospitalization, oral steroids, physiotherapy and NSAID whenever necessary. The initial dose of prednisolone consisted of 40-60 mg daily, depending on the severity of the reaction, gradually tapering off by 5-10 mg every 2-4 weeks in the case of type 1 reaction and by 5-10 mg every 1-2 weeks in the case of type 2 reaction. The dose of clofazimine was increased to 300 mg daily in all cases of erythema nodosum leprosum (ENL) which was gradually tapered to 200 mg and then to 100 mg daily depending upon the response of the patients. Treatment of trophic ulcer was carried out as usual after admitting the patient to the hospital. Microcellular rubber (MCR) sandals were provided to all affected patients, and they were educated to take care of anesthetic hands and feet. Treatment of eye complications was carried out as per the advice of the ophthalmologist; however, the disabilities reported in this communication do not include ocular deformities.
RESULTS
Table 1 depicts the impact of previous history of neuritis or reaction on their occurrence during treatment and post-treatment follow up. The patients have been segregated into two categories, those having a previous history of neuritis or reactions before commencement of therapy and those without any such history. In the vaccine group a statistically significant difference (p = 0.006) was observed in the incidence of fresh reactions (both type 1 and type 2) between the patients with a positive history of reactions compared to those without such history. The difference was significant within the placebo group as well (p <0.001).
The incidence of fresh neuritis was also found to be more in cases with a prior history of neuritis compared to those without such history. In the vaccine group, the incidence was 88.9% and 25.2% in the two categories, respectively (p <0.001), while in the placebo group the incidence was 72.7% and 33.8%, respectively (p = 0.005). In the two patient categories (with or without a previous history of neuritis), the comparison between the vaccine and the placebo groups was not significant statistically (p = 0.132 and p = 0.058).
Table 2 shows the incidence and course of peripheral anesthesia in the two patient groups. Resolution of anesthesia by more than 50% of the surface area involved has been considered as improved, while resolution of anesthesia by less than 50% of the surface area involved was considered as partial improvement. As it is evident from the table, the initial overall incidence was similar in the vaccine and placebo groups and also in the three leprosy types individually. However, a statistically significant difference was observed in LL patients, with more patients in the vaccine group showing improvement as compared to the placebo group which had a significantly higher number of patients with partial improvement. Two LL cases, one each in the vaccine and placebo groups, showed an extension of the area of anesthesia.
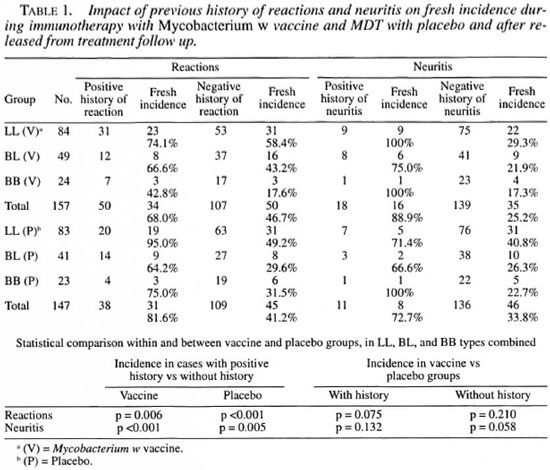
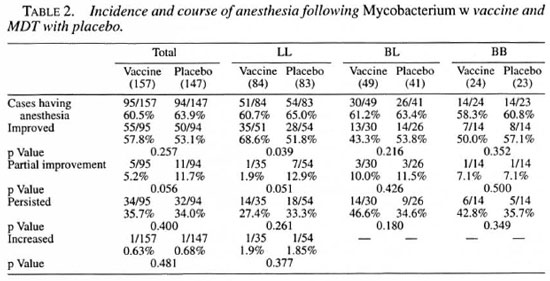
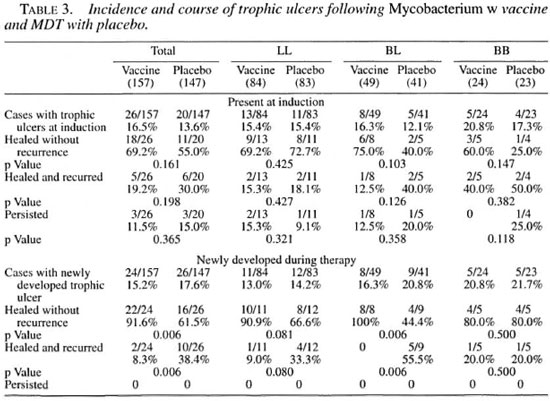
Table 3 gives an account of the incidence and course of trophic ulcers present at the time of induction and acquired during the course of treatment. There was no significant difference between the vaccine and placebo groups with respect to recurrence or healing of ulcers present at the time of induction. However, a statistically significant difference was observed in the course of healing of newly acquired ulcers; 91.6% of cases in the vaccine group showed healing without recurrence as compared to 61.5% in the placebo group (p = 0.006). More cases from the placebo group showed recurrence of trophic ulcers following healing, 38.4% against 8.3% in the vaccine group (p = 0.006). No case in either group showed persistence of ulcer.
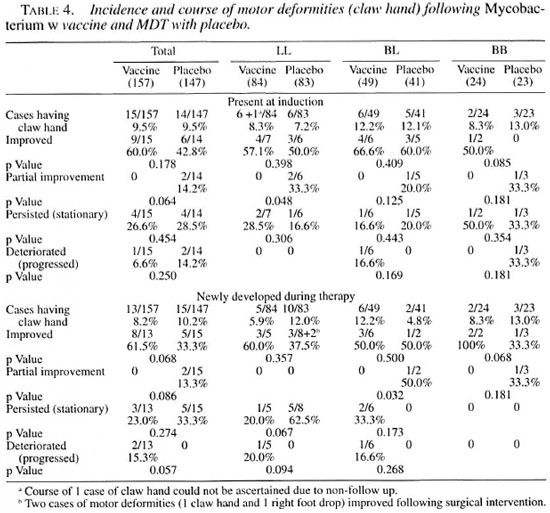
Table 4 shows the incidence and course of motor deformities present at the time of induction and acquired during the course of treatment and follow up. By far the most common motor deformity was claw hand. For the sake of brevity, details such as unilateral, bilateral, ulnar, median claw, etc., are not discussed here. The overall incidence of motor deformities was comparable in the two groups at induction without any statistically significant difference. The incidence of fresh deformities was comparable to those present at induction, in both the vaccine and placebo groups. Comparisons of improving, stationary or deteriorating motor deformities did not show any statistically significant differences in the vaccine and placebo groups.
Table 5 shows the incidence of permanent deformities, such as minimal digital absorption (grade 2) and other grade 3 deformities such as nasal collapse, permanent fixed claws and digital amputation, etc., as observed before and after treatment. For these deformities no statistically significant difference in the number of cases was observed in the two groups. However, the incidence of newly developed grade 3 deformities was significantly lower as compared to that at induction within the vaccine (p = 0.001) and the placebo group (p = 0.0001).
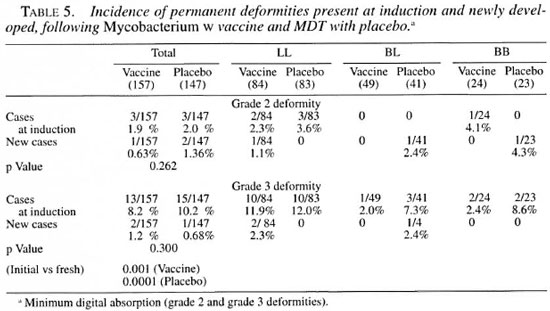
The crude deformity rate (excluding the invisible, i.e., grade 1 deformities) at the time of induction (before treatment) was 32.4% (51/157) in the vaccine group and 28.6% (42/147) in the placebo group. The corresponding figures for the new incidence during treatment and post-treatment follow up in the two groups were 24.8% (39/157) and 26.5% (39/147), respectively. The total observation periods (inclusive of MDT and follow-up surveillance duration) in the two groups were 997.3 years (per patient average 6.35 years) in the vaccine group and 899.1 years end (average 6.11 years) in the placebo group. The crude fresh deformity incidence was 51.3 and 46.7 per 1000 person-years of observation in the vaccine and placebo groups, respectively.
DISCUSSION
Prevention of disability is one of the objectives of leprosy eradication programs. Therefore it seems relevant to know what percentage of newly registered patients develop new disabilities or improvement/deterioration of existing disabilities during and after completion of MDT as well as immunotherapy. Results of different studies elsewhere on the effect of MDT on disability have yielded widely varying observations.
The incidence of type 1 reaction was found to be higher in those patients receiving the Mycobacterium w vaccine, especially in the LL patients where there was a statistically significant difference in comparison to the placebo group. This provides an indirect evidence of the promising immunotherapeutic potential of Mycobacterium w vaccine (data not shown). At the same time, it is satisfying to observe that this high occurrence of type 1 reaction is not leading to any higher incidence of deformities, if the reactions are treated early enough and appropriately.
The healing of pre-treatment trophic ulcers (present at induction) was slightly higher in the vaccine group than in the placebo group although not significant statistically. But the healing rate in post-treatment ulcers was statistically significantly higher in the vaccine group. Healing of trophic ulcers occurs irrespective of change in the status of anesthesia. However, with the associated recovery of anesthesia, the chances for the recurrence of ulcers after healing seem to be reduced. MDT seems to play an indirect role by restricting nerve damage through bacillary clearance. However, other factors also play a major role, e.g., care of parts, antibiotics, rest, local hygiene, management of associated conditions like diabetes, appropriate footwear, etc.
The vaccine group showed a slightly higher rate of recovery of motor impairments (mainly claw hand) as compared to the placebo group, although the difference was not statistically significant. The observation was valid both for deformities present at induction and also for those acquired during therapy. A remarkable reduction in the posttreatment incidence of grade 3 deformities, as compared with pre-treatment incidence, observed in both the vaccine and placebo groups, points toward the definitive role of MDT in arresting the progression of primary deformities like nasal collapse through timely chemotherapeutic intervention.
An interesting observation among the patients receiving the Mycobacterium vr vaccine in addition to MDT, and with no previous history of neuritis, was the slightly lower incidence of neuritis during treatment and surveillance in comparison to their counterparts in the placebo group. However, the difference is not significant statistically, suggesting that immunotherapy with Mycobacterium w does not lead to any excessive nerve damage as compared to that observed with MDT with the placebo. A similar observation was also made by Convit, et al . (2) during immuno-chemotherapy studies with BCG + M. leprae when the cases receiving immunotherapy had fewer episodes of neuritis than the controls. The clinical observations of slightly higher rates of recovery of sensory and motor deficits of the nerves in those patients receiving immunotherapy along with MDT is also in keeping with the histological observation reported earlier by our group, that after vaccination with the Mycobacterium vr vaccine no damage or increased intra/perineural lymphocytic infiltration was seen in the dermal nerve twigs (6).
Early diagnosis of the disease, adequate and effective treatment of the case, and early recognition and effective management of neuritis and reactional phenomenon constitute the best strategies for primary prevention of impairment (8). This study indicates that even in patients who present with peripheral sensory loss or other early disabilities and in whom these impairments have occurred during treatment, not only is its recovery possible but further deterioration can be prevented by proper and regular monitoring of the patients and timely intervention for the neuritis and reactions as and when they develop. The use of corticosteroids in appropriate doses over a period of 3-6 months is reported to prevent the establishment of nerve trunk paralysis in a high proportion of cases when the condition of quiet nerve paralysis is identified and treated before the nerve damage becomes irreversible. The recovery of claw-hand deformities, in the present study, highlights the importance of the early recognition and prompt and adequate treatment with steroids, analgesics and physiotherapy. In addition to these factors, the role of health education for the patients cannot be underestimated in the prevention of secondary impairments. Only through health education can the patients protect their insensitive parts from injury, get any injury healed early, maintain supple joints and be on the lookout for signs of onset or progress of nerve damage (11).
CONCLUSION
The occurrence of reactional episodes and neuritis in multibacillary leprosy following the addition of the Mycobacterium w vaccine to MDT is nearly equal to that observed with MDT alone.
There is a markedly reduced occurrence of reactional episodes and neuritis during therapy and follow up in patients not having a previous history of the episodes in comparison to those with a prior history.
The incidence of all grades of deformities (except grade 3), e.g., anesthesia, trophic ulcers, motor deformities, shows no difference in the vaccine and placebo groups before or after MDT. The course of deformities (healing of trophic ulcers and recovery of claw hands) shows a slightly better outcome in the vaccine group in comparison to the placebo group, although the vaccine may not be directly responsible for this.
The incidence of primary deformities such as saddle nose is reduced significantly after starting MDT and during follow up in both the vaccine and placcbo groups.
Acknowledgment. These clinical trials were supported by a grant from the Department of Biotechnology, Ministry of Science and Technology, the Government of India. Dr. Padam Singh, Director, Institute of Research in Medical Statistics, New Delhi, is gratefully acknowledged for his help in the statistical analysis of the data. The technical assistance of Mr. Dinesh Negi, Mr. Amarnath Prasad and Mr. Anil Bobin in the day-to-day work of the clinics is also gratefully acknowledged. Last, but not least, the authors feel immensely indebted to the patients of this study without whose support and cooperation such a long study would not have been possible.
REFERENCES
1. Brandsma, J. W., de Jong, N. and Tjepkema, T. Disability grading in leprosy; suggested modifications to the WHO disability grading form. Lepr. Rev. 57 (1986) 361-369.
2. Convit, J., Aranzazu, N., Ulrich, M., Pinardi, M. E., Reyes, O. and Alvarado, J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsudanegative contacts. Int. J. Lepr. 50 (1982) 415-424.
3. Gilbody, J. S. Aspects of rehabilitation in leprosy. Int. J. Lepr. 60 (1992) 608-640.
4. Goodwin, C. S. The use of voluntary muscle testing in leprosy neuritis. Lepr. Rev. 39 (1968) 209-216.
5. Iyere, B. B. Leprosy deformities: experience in Molai Leprosy Hospital, Maiduguri, Nigeria. Lepr. Rev. 61 (1990) 171-179.
6. Mukherjee, A., Zaheer, S. A., Sharma, A. K., Misra, R. S., Kar, H. K., Mukherjee, R. and Talwar, G. P. Histopathological monitoring of an immunotherapeutic trial with Mycobacterium w . Int. J. Lepr. 60 (1992)28-35.
7. Sehgal., V. N. and Sharma, P. K. Pattern of deformities and disabilities in urban leprosy. Indian J. Lepr. 57 (1985) 183-192.
8. Sharma, P., Kar, H. K., Bi:i;na, K. R., Kaur, H. and Narayan, R. Disabilities in multibacillary leprosy patients: before, during and after multidrug therapy. Indian J. Lepr. 68 (1996) 127-136.
9. Sharma, P., Kar, H. K., Misra, R., Mukherjee, A., Kaur, H., Mukhhkjix, R. and Rani, R. Induction of lepromin positivity following immunochemotherapy with Mycobacterium w vaccine and multidrug therapy and its impact on bacteriological clearance in multibacillary leprosy; a report on a hospital-based clinical trial with the candidate antileprosy vaccine. Int. J. Lepr. 67 (1999) 259-269.
10. Smith, W. C. S., Antin, V. S. and Patole, A. P. Disability in leprosy; a relevant measurement of progress in leprosy control. Lepr. Rev. 51 (1980) 155-166.
11. Srinivasan, H. Not by chemotherapy alone. Indian J. Lepr. 66 (1994) 209-221.
12. Talwar, G. P., Zaheer, S. A., Mukherjee, R., Wai.ia, R., Misra, R. S., Sharma, A. K, Kar, II. K, Mukherjee, A., Parida, S. K., Suri:sh, N. R., Nair, S. K and Pandi-y, R. M. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium ir, in multibacillary leprosy patients. Vaccine 8 (1990) 121-129.
13. WHO Study Group. Chemotherapy of leprosy for control programme. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
14. Zaheer, S. A., Bi:i:na, K. R., Har, H. K., Sharma, A. K., Misra, R. S., Mukhhrjki;, A., Mukhkkjhi:, R., Kaur, H., Pandhy, R. M., Wai.ia, R., Mukhopadhyay, A. and Talwar, G. P. Addition of immunotherapy with Mycobacterium w vaccine to MDT benefits multibacillary leprosy patients. Vaccine 13 (1995) 1102-1110.
1. D.V.D., National Institute of Immunology, New Delhi 110 067, India.
2. Ph.D., National Institute of Immunology, New Delhi 110 067, India.
3. Ph.D., National Institute of Immunology, New Delhi 110 067, India.
4. Ph.D., National Institute of Immunology, New Delhi 110 067, India.
5. M.D., Department of Dermatology, Venereology and Leprology, Dr. Ram Manohar Lohia Hospital, New Delhi 110 001, India.
6. M.D., Department of Dermatology and Leprology, Safdarjung Hospital, New Delhi 110 029, India.
7. M.D., Institute of Pathology, Indian Council of Medical Research, Safdarjung Hospital Campus, New Delhi 110 029, India.
Reprint requests to Dr. Pankaj Sharma, Neuroimmunology Division, National Institute of Immunology, New Delhi 110 067, India. FAX: 91-11-616-2125; e-mail: rajni@nii.res.in
Received for publication on 9 November 1998.
Accepted for publication in revised form on 29 June 1999.