- Volume 67 , Number 3
- Page: 259–69
Induction of lepromin positivity following immuno-chemotherapy with Mycobacterium w vaccine and multidrug therapy and its impact on bacteriological clearance in multibacillary leprosy: Report on a hospital-based clinical trial with the candidate antileprosy vaccine
ABSTRACT
A vaccine based on autoclaved Mycobacterium w was administered, in addition to standard multidrug therapy (MDT), to 157 bacteriologically positive, leprominnegative, multibacillary (LL, BL and BB) leprosy patients. The vaccinees were supported by a well-matched control group of 147 patients with similar type of disease who received a placebo injection in addition to MDT. The MDT was given for a minimum period of 2 years and continued until skin-smear negativity, while the vaccine was given at 3-month intervals up to a maximum of 8 doses. The lepromin response evaluated in terms of percentage of subjects converting to positivity status, measurement in millimeters, and duration of lepromin positivity sustained, reflected a statistically significant better outcome in the vaccine group patients (especially LL and BL leprosy) in comparison to those in the placebo group. The data indicate that lepromin-positivity status seems to have an impact on accelerating the bacteriological clearance, as is evident by the statistically significant accelerated decline in the BI of those patients who converted to lepromin positivity as compared to those remaining lepromin negative throughout therapy and post-therapy follow up. To conclude, the addition of the Mycobacterium w vaccine to standard MDT induces a lepromin response of a statistically significant higher magnitude than that observed with MDT alone.RÉSUMÉ
Un vaccin, préparé à partir de Mycobacterium w, autoclavées, fut adminstré à 157 patients hanséniens multibacillaires (LL, BL et BB), positifs à l'examen bactérioscopique et négatifs au test de la lépromine, en même temps que la poly-chimiothérapie (PCT) standard. Les vaccinés ont été comparés à un groupe contrôle similaire dc 147 patients présentant le type correspondant de maladie hansénienne, et qui ont reçu une injection placebo en plus de la PCT. La PCT fut adminstrée pendant une période minimale de 2 années et prolongée jusqu'à ce que l'examen de suc dermique fût négatif, tandis que la vaccin fut administré touse les 3 mois, avec un maximum de 8 doses. La réponse au test de la lepromine a été évalué;aae par le pourcentage de sujets évoluant vers un statut positif, la taille en millimètres et la durée du statut de positivité. Il a été constaté qu'elle traduisait une meilleure issue, statistiquement significative, chez le groupe de patients vaccinés (en particulier les sub-groups BL et LL) par rapport au groupe placebo. Les données suggèrent que le statut positif au test de la lépromine semble avoir un impact positif sur la clairance bactériologique, comme l'indique la diminution accélérée, de façon statistiquement significative, de l'index bactériscopique chez les patients qui ont évolué vers un test positif à la léprominc en comparaison de ceux qui sont restés lépromine-négatifs durant et après le traitement. Pour conclure, l'addition d'un vaccin Mycobacterium w . en complément de la PCT standard induit une réponse statistiquement plus importante au test de la léprominc que la réponse observée avec la seule PCT.RESUMEN
Se administró una vacuna autoclaveada de Mycobacterium w; además de la poliquimioterapia (PQT) estándar, a 157 pacientes con lepra bacteriológicamente positivos, lepromina negativos y multibacilares (LL, BL y BB). Un grupo similar de pacientes recibió sólo placebo además de la PQT. La PQT se administro por un periodo minimo de 2 anos y se continuo hasta que los extendidos de linfa cutânea se hicieron negativos; la vacuna se administro a intervalos de 3 meses hasta un máximo de 8 dosis. La respuesta a la lepromina (en mm), evaluada en términos dei porcentaje de indivíduos convertidos a positivios, y la duración de la positividad a la lepromina, fueron superiores en los pacientes vacunados (especialmente en los casos LL y BL) que en el grupo tratado con placebo. Los datos indican que el estado de positividad a la lepromina parece influir positivamente en la depuración de los bacilos de la lepra. La cinética de depuración, establecida por medición dei BI, lue estadisticamente más acelerada en los pacientes convertidos a leprominopositivos que en los pacientes que siguieron siendo lepromino-negativos durante la terapia y después de ella. En conclusion, la adición de Mycobacterium w a la PQT estándar, induce una mayor frecuencia de conversion a la lepromino-positividad que la PQT sola.The lepromin response, which is a hypersensitivity response, indicates the status of cellular immunity against Mycobacterium leprae in leprosy patients. Lepromin negativity in lepromatous leprosy (LL) is indicative of the lack of host cellular immune response to M. leprae infection (6). Hypersensitivity reactions to the intradermal injection of killed M. leprae (lepromin response) could be subdivided into two types: an early (Fernandez) reaction read at 24-48 hours, a tuberculin-like delayedtype hypersensitivity (DTH) reaction (Type IV reaction of Coombs and Gel classification) measured by the area of erythema and induration; and the late (Mitsuda) reaction (Type VI Coombs and Gel classification) read after 3-4 weeks, an induration of skin caused by granuloma formation. The early reaction which is observed following the administration of soluble antigen preparations (leprolins/leprosins) is an indicator of pre-existing hypersensitivity. The soluble preparations of M. leprae do not themselves lead to sensitization of the recipient; the late response, however, is a reflection of sensitizing capability of the antigen (micro-vaccination) (5). The intact lepra bacilli preparations (lepromins) elicit the early as well as the late response. The significance of the two types of lepromin reactions has been reported to be the same, an observation confirmed by Fernandez (8) and Dharmendra and Lowe (7). The lepromin test as such remains an important skin test in use as a surrogate marker of the acquisition of a cell-mediated immune response to M. leprae antigen following immune boosting therapy, although reservations have been expressed about its applicability as a foolproof criterion (9).
A vaccine based on Mycobacterium w bacilli has been under evaluation for its immunotherapeutic effects as an adjunct to multidrug therapy (MDT) in a hospitalbased trial in Delhi, India, since 1987 (19). The vaccine was administered along with MDT to lepromin-negative, multibacillary (MB) leprosy cases, predominantly with a high pre-treatment bacterial index (BI). The control group was a well-matched group of patients who received a placebo injection in place of the vaccine along with MDT. Patients were followed up after completion of vaccine administration for a period of 5 years. One of the objectives of this study was to evaluate the immunizing potential of the Mycobacterium w immunomodulator, as monitored in terms of lepromin conversion from negativity to positivity status (delayed Mitsuda response), and its impact on bacteriological clearance which is reported in this communication.
MATERIALS AND METHODS
Vaccine. A suspension of killed Mycobacterium w in physiological saline was used as the vaccine. The details of the vaccine preparation have been reported earlier (19). The first dose of vaccine was 1 x 109 autoclaved bacilli in 0.1 ml physiological saline (0.85% NaCl). Subsequent doses contained half the number of bacilli, i.e., 5 x 108. The vaccine was administered intradermally in the deltoid region. A total of eight doses were given at 3-month intervals over a period of 2 years.
Placebo. One gram of micronized starch (Sarabhai Chemicals, Baroda, India) was dissolved in 100 ml of distilled water, autoclaved at 15 lb per square inch pressure for 15 min and dispensed in sterile vials.
Lepromin-A and lepromin test. Armadillo-derived lepromin containing 30-40 million killed bacilli per ml was kindly made available by IMMLEP/TDR of the World Health Organization (WHO) (Lot No. C-l; preparation date 6/14/89; NHDC, Carville, Louisiana, USA). After commencement of therapy the patients were tested with 0.1 ml of lepromin-A administered intradermally on the mid-volar region of left forearm in a lesion-free area of the skin. The response was read after 4 weeks and retesting was done every 3 months. The size of the delayed response (Mitsuda reaction) was recorded in millimeters.
Multidrug therapy (MDT). In the initial phase, MDT consisted of 2 weeks of intensive therapy with 600 mg of rifampin, 100 mg of clofazimine and 100 mg of dapsone daily. Subsequently, the patients received the WHO-recommended regimen of 600 mg of rifampin and 300 mg of clofazimine once a month, supervised, plus 100 mg of dapsone and 50 mg of clofazimine daily, self-administered. The MDT was given for a minimum period of 2 years and continued thereafter until skin smear negativity was attained.
Subjects and study design. Permission was obtained from the Institutional Ethics Committee and the Drug Controller General of India before initiating the study and informed consent of the subjects was taken before inducting them into the trial. Induction of patients was initiated in November of 1986 and concludcd in May of 1992. The enrolled subjects comprised untreated, lepromin-negative, bacteriologically positive, active cases of MB leprosy patients of the LL, BL and BB types. These patients, who were deficient in cell-mediated immunity against M. leprae (as evident by their lepromin negativity and high bacteriological positivity in slit-skin smears), were chosen so that the immuno-modulatory effects of the vaccine could be critically assessed in terms of the boost in cell-mediated immunity and bacterial clearance. The diagnosis was established on the basis of clinical examination and histopathology. Standard MDT was administered to all patients inducted into the trial; however, in addition, the vaccine group received the vaccine whereas the control group was given the injection of micronized starch as a placebo.
The ongoing clinical trials had two series of cases. The first series (single blind) was composed of 120 MB leprosy patients and the vaccine codes were known to the head of the clinic but not to the attending clinicians. The second (double blind) series of the trial was composed of 350 MB patients but neither the evaluating agency nor the attending clinicians were aware of the identity of the injection administered. To ensure blinding, the upper part of the arm, i.e., the vaccination/placebo site, was covered with a cloth napkin by the nonmedical assistant before the patient was examined by the medical officer. The same procedure was adopted while recording the lepromin response. The slides for BI smears were prepared by the paramedical staff in the clinic and were coded with numbers which were subsequently read by the medical officer at the National Institute of Immunology without any clue as to the identity of the vaccine codes. The vaccine codes were opened in 1994 for the purpose of analyses of the data, after which the data from both series were combined since the protocol followed for treatment and follow up in the two series was similar and the parameters of monitoring were identical. However, for the follow up in the clinics, the blinding procedures mentioned above were exercised during clinical examinations even after decoding.
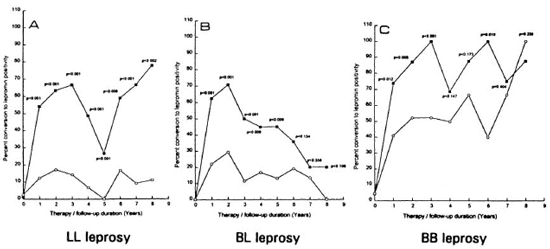
Fig. 1. Percent conversion to lepromin positivity among vaccine ( ) and placebo (
) and placebo ( ) groups in LL (A), BL(B) and BB (C) leprosy; p values indicate statistically significant difference between vaccine and placebo groupsat respective time points.
) groups in LL (A), BL(B) and BB (C) leprosy; p values indicate statistically significant difference between vaccine and placebo groupsat respective time points.
Statistical analysis. The statistical analyses for the comparison of durations of lepromin positivity in the two groups were done using the Student's t test. The percentages of patients converting from negativity to lepromin positivity were compared using a Z test for two proportions from two independent groups. The comparison of response (in mm) and duration of sustained positivity were done using the Student's t test. The data with p <0.05 were considered as significant.
RESULTS
The lepromin status of the patients was monitored periodically for a period of 8 years which included a 2-year period of vaccine or placebo administration and 6 years of post-placebo/vaccination follow up. Out of 422 patients inducted, 109 cases (25.8%) defaulted treatment at various stages. Such cases were evenly distributed between the vaccine and placebo groups (27.8% and 23.4%, respectively); 77 (70.6%) of the defaulters abandoned treatment after the initial few (<9) months, falling under the category of "false dropouts," the reference assigned to patients who discontinue treatment for reasons such as carelessness, apathetic attitude toward self and/or inability to travel long distances for treatment for long durations. Such cases may continue treatment from another treatment center (13). However, 32 (29.4%) out of 109 patients defaulted treatment after 10 or more months of therapy, thus bringing the effective drop-out rate to 32/422 (7.5%) with a nearly equal distribution between vaccine and placebo cases (7.8% and 7.2%, respectively).
The comparative analyses between the vaccine and placebo groups were carried out to determine: a) the percentage of cases who converted to positivity from lepromin negative status; b) size of induration (in mm) of lepromin response at different time points of therapy; c) period of sustained lepromin positivity status following 6-8 doses of vaccine or placebo administration; and d) association of bacteriological clearance and lepromin status.
Conversion to lepromin positivity. The percentages of the LL, BL and BB cases who converted to lepromin positivity from the negative status with 2 years of chemoimmunotherapy are shown in Figure 1. At the 2-year time point, 63.4% of LL, 70.8% of BL and 87.0% of BB leprosy patients in the vaccine group had converted to lepromin positivity as compared to 17.3% of LL, 29.3% of BL and 52.2% of BB patients in the placebo group, and this difference was statistically significant. The difference in the percent lepromin positivity between the vaccine and placebo groups remained statistically significant until 8 years of follow up in the LL group, until 5 years of follow up in the BL group; and 3 years of follow up in the BB group. During the post-vaccination/placebo follow up, the percent positivity in the LL and BB patients continued to remain high, while in BL patients there was a decline observed in percent positivity, although the statistical difference between the two groups remained significant.
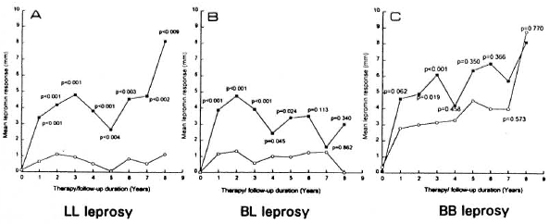
Fig. 2. Comparison of lepromin response in millimeters at different time points between vaccine ( ) andplacebo (
) andplacebo ( ) groups for LL (A), BL (B) and BB (C) leprosy; p values show statistical comparison at respectivetime points. Each point is the mean of induration size of ali the patients in the group (vaccine or placebo).
) groups for LL (A), BL (B) and BB (C) leprosy; p values show statistical comparison at respectivetime points. Each point is the mean of induration size of ali the patients in the group (vaccine or placebo).
Lepromin induration. Figure 2 depicts the comparison of the lepromin responses in terms of measurements in the two groups studied in the three leprosy types. Calculation of the mean induration size which included all of the lepromin-positive and -negative cases combined in the vaccine and placebo groups at different time points, and the statistical significance between the two group was determined. From year 1 onward the mean lepromin response showed wide differences in all three categories (e.g., the mean measurement at year 1 for the LL patients was 3.38 mm in the vaccine group and 0.64 mm in the placebo group. As evident from the p values, the levels of statistically significant differences of the lepromin responses between the vaccine and placebo groups was maximum in LL patients (observed until year 8) followed by slightly lower levels in BL (observed until year 5) and further lower levels in BB patients (observed until year 3).
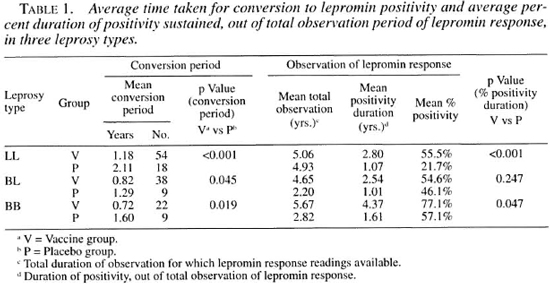
Duration of lepromin positivity. Table 1 shows the comparison of the average time it took patients in the two groups to convert to lepromin-positive status from negativity, as well as the duration of positivity as a percent of the total duration of observation of the lepromin status. The mean conversion period was significantly shorter in the vaccine group as compared to the placebo group for all three leprosy types. Similarly, the durations of percent positivity were longer in the vaccine group as compared to placebo in the LL type, and this difference was highly significant (p <0.001) statistically although the difference was marginally higher (p = 0.047) in BB patients.
Table 2 depicts the durations for which lepromin positivity induced by immunochemotherapy and chemotherapy was sustained in the different leprosy types. For this purpose the cases were classified into three categories according to the lepromin status: a) sustained positive = those who exhibited a lepromin positive response for a continuous period of at least 6-9 months and more; b) negatives = those cases who remained lepromin negative throughout therapy and during follow up; and c) fluctuating = those who showed a fluctuating pattern of positivity and negativity within a period of less than 1 year.
It is evident from Table 2 that the number of patients with sustained lepromin positivity in the vaccine group was higher than in the placebo group for all three leprosy types. The pooled estimation of lepromin positivity duration among the sustained positives and negatives, when all cases are considered together, shows the statistical difference between the vaccine and placebo groups more clearly-p <0.001 in LL, p = 0.001 in BL and p = 0.013 for BB patients. In the fluctuating group, the statistically significant difference (p <0.012) in the duration of lepromin positivity was observed only in LL patients, i.e., more patients in the vaccine group showing fluctuating lepromin response than in placebo group.
Correlation between bacteriological clearance and lepromin status. An in depth analysis of the lepromin data was carried out to study the correlation, if any, between BI clearance and lepromin status. Figures 3A and 3B present the mean cumulative BI falls, shown at different time points, in the lepromin-positive and -negative cases in the vaccine and placebo groups for LL and BL leprosy types, respectively.
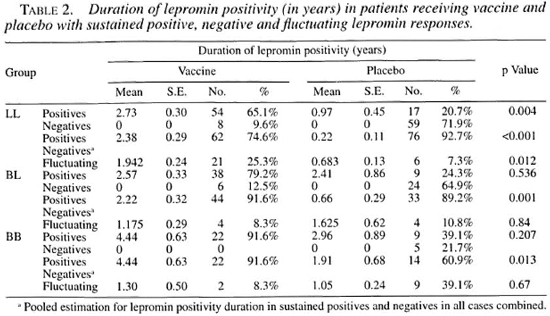
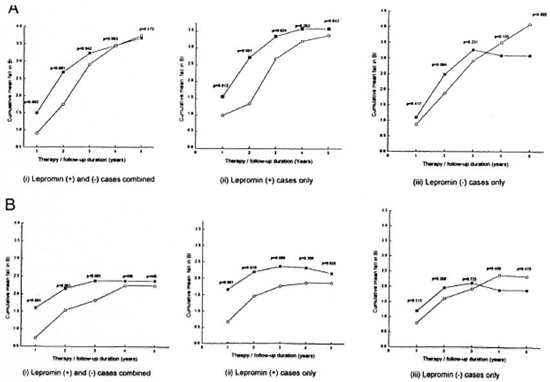
Fig. 3A. Correlation of lepromin status with mean fali in 131 (cumulative) in LL leprosy among (i) lepromin-positive and -negative cases combined, (ii) lepromin-positive cases only, and (iii) lepromin-negative cases onlyin vaccine ( ) and placebo (
) and placebo ( ) groups.
) groups.
Fig. 3B. Correlation of lepromin status with mean fali in BI (cumulative) in BL leprosy among (i) lepromin-positive and -negative cases combined, (ii) lepromin-positive cases only, and (iii) lepromin-negative cases onlyin vaccine ( ) and placebo (
) and placebo ( ) groups.
) groups.
Figure 3A shows the BI decline in LL patients. As is evident from Figure 3A (i), there is a statistically significant difference in BI decline in the vaccine and placebo groups until the 3-year time point when all the cases are taken together. However, when a graph is plotted taking into account only the cases that converted to lepromin positivity, the gap between the vaccine and placebo groups' curves is further widened, showing the significance of the difference between the two groups (also corroborated by the lower p values). The lepromin-negative cases did not show any significant difference in the BI fall in either the vaccine or the placebo group, thus indicating the relationship between conversion to lepromin positive status and faster rate of BI decline. A similar trend was observed in BL leprosy (Fig. 3B). In the BB leprosy patients the difference in the decline in the BI was not significant in either the vaccine or the placebo group, probably due to very low BI levels in both groups (data not shown). The comparison between lepromin-positive and -negative cases was not possible because all patients in the vaccine group converted to lepromin positivity.
Reactional episodes, neuritis and deformities. The overall incidence of type 1 reaction was 30.5% and 19.7% in the vaccine and placebo groups, respectively. The incidence of type 2 reactions in the two groups was 31.8% and 34.6%, and that for neuritis was 32.4% and 36.7% in the vaccine and placebo groups, respectively. None of the differences was statistically significant except for a higher incidence of type 1 reactions (mild episodes of upgrading reactions associated with lepromin conversion) in the LL vaccine group patients (29.7%) as compared to a 12.0% incidence in LL placebo patients (p = 0.002).
The incidence of disabilities such as anesthesia, trophic ulcers, claw hand and grade 3 deformities present before therapy and those developed during therapy and follow up was not different statistically in the vaccine and placebo groups (detailed analysis is being reported elsewhere in this issue (18).
DISCUSSION
The search for a suitable immunomodulator against M. leprae infection has led to the development of a number of candidate vaccines, some of which have reached advanced stages of clinical trials. The rationale behind the immunomodulatory intervening approach in leprosy was aimed at a) immunotherapeutic benefits, i.e., acceleration of bacteriological clearance and resolution of lesions in general, and b) immunoprophylactic potential, i.e., ability to induce sensitization leading to imparting protection against disease development following infection with M. leprae . For assessment of the immunoprophylactic potential of an immunomodulator in leprosy, the only rational method is the prospective study to look for the disease incidence following administration of the immunomodulator. The cases in such studies need to be followed up for an adequate duration of incubation period, i.e., 8-10 years (2). However, the lepromin reaction has long been considered a surrogate marker of the cell-mediated immune response and has served as a reliable tool for the assessment of the sensitizing potential of an immunomodulator. A positive lepromin response is generally considered beneficial in imparting resistance against the development of multibacillary leprosy, although exceptions have been reported in some studies where no correlation was observed between reactivity to M. leprae antigens and the protection imparted against disease development (4).
Histologically, the early lepromin response is an acute inflammatory reaction of the dermis and epidermis. There is an extravasation of fluid from congested capillaries causing induration, and the infiltrate is predominantly neutrophilic with scanty lymphocytes. The late response, on the other hand, is basically a tuberculoid type of reaction. The hallmark of this type of reaction is the presence of epithelioid cells, lymphocytes and the resemblances to a tuberculoid granuloma. The macrophages in lepromatous leprosy fail to eliminate the leprosy bacilli (instead, rapid multiplication of bacilli takes place) and they are unable to transform to epithelioid cells. This histological observation seen in lepromatous leprosy explains the histological basis of the lepromin-negative response (5).
Various studies have been done using different vaccines. However, the parameters studied by them for assessment of cell mediated immune responses vary. While some investigators have used lymphocyte transformation tests against M. leprae antigens and serum nitrite levels (16,17) to study the cell-mediated immunity, others have reported alteration in the lepromin response following immuno-chemotherapy. In a study from Venezuela in a group of MB patients given MDT and 8-10 doses of immunotherapy over 3 years, lepromin positivity to M. leprae soluble antigen (SA) was induced in 75% of patients after therapy as compared to 5% before therapy. Of these 75% of the subjects, 26% had a lepromin response of 10 mm or above (17). Spontaneous conversions to lepromin-A positivity following chemotherapy alone have been reported with conflicting observations. A conversion of 54% of the patients was observed in a group of lepromatous patients by Waters, et al. (22) while Majumdar, et al. (12) observed lepromin conversion in only 5% of the cases in MDT-treated patients. However, we found that 17.3% of LL patients on MDT converted to lepromin positivity at the 2-year time point as compared to 63.4% of the patients in the vaccine group. The in-vitro studies of Rada, et al. C6-17) show that MB patients treated with MDT did not acquire positive reactivity to M. leprae antigens during follow up, while those on immunotherapy with BCG plus heat-killed M. leprae showed high and long-lasting T-cell responses to mycobacterial antigens in a significant number of initially unresponsive MB patients. In another study using low-dose Convit vaccine with MDT in a group of LL patients, lepromin conversion following 6-8 doses was observed in 63.3% of the patients receiving the vaccine as compared to 5% of the patients receiving MDT alone. Also, the induced lepromin positivity was sustained only during vaccine administration and 55% of the cases reverted back to negativity after cessation of immunotherapy (l2). However, our data show that 65.1 % of the LL patients on vaccine had a sustained lepromin-positive status for a mean of 2.7 years, while 25.3% showed a fluctuating lepromin response.
Studies for the evaluation of lepromin sensitization potential with different candidate antileprosy vaccines, e.g., BCG plus killed M. leprae , ICRC and Mycobacterium w , have included subjects who were apparently healthy individuals and close contacts or noncontacts of leprosy patients (9-10,21). BCG has been shown to offer variable protection in different types of leprosy; however, these studies have been mainly conducted with borderline or a few LL cases (14-20). In contrast, the subjects in our study were untreated, multibacillary leprosy patients with a high initial BI and leprominnegative status. Hence, the mean values of the lepromin-reaction measurements are much lower than those observed in other studies with apparently healthy individuals. The lepromin status of patients from the vaccine and placebo groups shows a statistically significant difference in terms of patients converting to lepromin positivity and in the size of lepromin reaction, as well as sustained positivity over considerable periods of time in the vaccine group. In all three leprosy types (LL, BL and BB) the responses have been higher in the vaccine group as compared to the placebo group. However, a statistically significant difference between the vaccine and placebo groups (p value) was observed until year 8 in LL, year 5 in BL, and year 3 in BB patients which clearly demonstrates that the cell-mediated immunity to M. leprae antigen is boosted by the addition of the vaccine to MDT (evident from the poor upgrading of lepromin status in LL placebo cases). The difference between the two groups was maximum in LL patients whose inherent immune deficit is maximal; the gap between the vaccine and the placebo groups narrowed down in BL patients whose host cell-mediated immunity response is higher in comparison to LL, followed by BB patients whose inbuilt cell-mediated immunity is further elevated, thus narrowing down the difference between the two groups studied. These observations suggest that addition of the Mycobacterium w vaccine to MDT upgrades the cell-mediated immunity of patients to a significantly higher extent than that observed in cases receiving MDT alone. Katoch, et al. (11) also found a more rapid fall in the BI and killing of viable bacilli using the Mycobacterium w vaccine (as seen by mouse foot pad and bacillary ATP estimation).
Since a significantly higher number of patients on vaccine had converted to lepromin positivity and also showed fall in the BI, we wanted to study whether these two observations have any correlation. Faster clearance of bacilli in Iepromin-positive (converted) cases suggests a boost in the cell-mediated immunity, through an immunomodulator which is crossreactive with M. leprae , in otherwise anergic MB leprosy cases. Our data show a positive correlation between lepromin positivity and bacteriological clearance, the observation being supported by the accelerated rate of bacteriological clearance seen in patients who converted to lepromin positivity status in contrast with the comparatively slower rate in patients who did not convert to lepromin positivity. These observations suggest that conversion to lepromin positivity has an impact on BI clearance since the lepromin status is a direct indication of the cell-mediated immunity against M. leprae . It has been observed that macrophages can be made to respond to (or contain) M. leprae invasion by means of sensitization through a suitable immunomodulator which can stimulate the otherwise ineffective macrophages (3). Thus, apart from being a reflection of the prognostic indicator (prophylactic index), the lepromin response also throws some light on the basic mechanisms involved in bacillary clearance by macrophages. The rapid treatment of lepromatous leprosy may not be of much importance from epidemiological considerations but is definitely important from the patient's point of view.
Thus, it can be inferred that the lepromin positivity status can be treated as a marker of the bacillary elimination capability of macrophages in a leprosy case. The vaccine not only induces lepromin positivity in a significantly larger number of patients, but it also sustains it for a longer duration as compared to the patients receiving MDT plus placebo. The BI decline was correlated with the lepromin-positive status of the patients and was more or less similar in both the vaccine and placebo recipients who did not convert to lepromin positivity. These findings corroborate the established fact that bacillary load in leprosy cases bears an inverse relationship with cell-mediated immunity status. However, the immunotherapy with Mycobacterium w did not precipitate type 2 reactions or neuritis over and above MDT; similar findings have been reported earlier by Katoch, et al. (11) using BCG and the Mycobacterium w vaccine.
Total duration of observation for which lepromin response readings available. J Duration of positivity, out of total observation of lepromin response.
Acknowledgment. The trials were supported by the Department of Biotechnology, Ministry of Science and Technology, Government of India. The authors wish to express their gratitude to Dr. Padam Singh, Director, Institute for Research in Medical Statistics (IRMS, ICMR), New Delhi, for his able guidance and Dr. Abha Aggarwal (IRMS, ICMR) for her sustained hard work in carrying out the statistical analysis of the data. The paramedical and technical assistance of Mr. Anil Bobbin, Mr. Dinesh Negi and Mr. Amarnath Prasad are gratefully acknowledged. Above all, the authors feel immensely indebted to the patients of this study, without whose support and cooperation such a long study would not have been possible.
REFERENCES
1. Chaudhury, S., Hajra, S. K., Mukherjee, A., Saha, B., Majumdar, V., Chattapadhya, D. and Saha, K. Immunotherapy of lepromin-negative borderline leprosy patients with low-dose Convit vaccine as an adjunct to multidrug therapy; a 6-year follow-up study in Calcutta. Int. J. Lepr. 65 (1997)56-62.
2. Convit, J., Aranzazu, N., Ulrich, M., Zuniga, M., de Aragon, M. E., Alvarado, J. and Reyes, O Investigations related to the development of a leprosy vaccine. Int. J. Lepr. 51 (1983) 531-539.
3. Convit, J., Pinardi, M. E., Rodriguez-Ochoa, G., Ulrich, M., Avii.a, J. L. and Goiiiman-Yaiir, M. Elimination of Mycobacterium leprae subsequent to local in vivo activation of macrophages in lepromatous leprosy by other mycobacteria. Clin. Exp. Immunol. 17 (1974) 261-265.
4. Convit, J., Sampson, C. and Zuniga, M. Immunoprophylactic trial with combined Mycobacterium leprae /BCG vaccine against leprosy: preliminary results. Lancet 1 (1992) 446-450.
5. Dharmendra. The lepromin test. In: Leprosy Vol. 2. Bombay: Samant & Co., 1985, pp. 999-1063.
6. Dharmendra and Chatterjee, K. R. Prognostic value of lepromin test in contacts of leprosy cases. Lepr. India 27 (1955) 149-157.
7. Dharmendra and Lowe, J. Studies of the lepromin test (6). Results of Mitsuda test in cases of leprosy of different clinical types. Lepr. India 14 (1942)3-12.
8. Fernandez, J. M. M. The early reaction induced by lepromin. Int. J. Lepr. 8 (1940) 1-8.
9. Gupte:, M. D., Anantharaman, D. S., de Britto, R. L. J., Vallishayee, R. S., Nagaraju, B., Kannan, S. and Sengupta, U. Sensitization potential and reactogenicity of BCG with and without various doses of killed Mycobacterium leprae . Int. J. Lepr. 60 (1993) 340-352.
10. Gupte, M. D., Vallishayee, R. S., Anantharaman, D. S., de Britto, R. L. J. and Nagaraju, B. Sensitization and reactogenicity of two doses of candidate antileprosy vaccine Mycobacterium \r. Indian J. Lepr. 68 (1996) 315-324.
11. Katoch, K, Katoch, V. M., Natrajan, M., Bhatia, A. S., Sreevatsa, Gupta, U. D., Sharman, V. D., Shivannavar, C. T., Path., M. A. and Bhardwaj, V. P. Treatment of bacilliferous BL/LL cases with combined chemotherapy and immunotherapy. Int. J. Lepr. 63 (1995) 202-211.
12. Majumdar, V., Mukherjee, A., Hajra, S. K., Saiia, B. and Saha, K. Immunotherapy of far-advanced patients of lepromatous leprosy with lowdose Convit vaccine along with MDT (Calcutta trial). Int. J. Lepr. 64 (1996) 26-36.
13. Naik, S. S. and Nore, P. R. The pattern of "dropouts" of smear-positive cases at an urban leprosy center. Indian J. Lepr. 68 (1996) 161 -166.
14. Ponnighaus, J. M., Fine, P. E. M., Sterne, J. A. C., Wilson, R. J., Msosa, E., Gkuer, P. J. K, Jenkins, P. A., Lucas, S. B., Liomba, N. G. and Buss, L. Efficacy of BCG vaccine against leprosy and tuberculosis in north Malawi. Lancet 1 (1992) 636-639.
15. Rada, E. M., Convit, J., Ui.rich, M., Gali.inotto, M. E. and Aranzazu, N. Immunosuppression and cellular immunity reactions in leprosy patients treated with a mixture of Mycobacterium leprae and BCG. Int. J. Lepr. 55 (1987) 646-650.
16. Rada, E., Ulrich, M., Aranzazu, N., Santaella, C., Gallinoto, M. E., Centeno, M., Rodriguez, V. and Convit, J. A longitudinal study of immunologic reactivity in leprosy patients treated with immunotherapy. Int. J. Lepr. 62 (1994) 552-558.
17. Rada, E., Ulrich, M., Aranzazu, N., Rodriguez, V., Centeno, M., Gonzalez, I., Santaella, C., Rodriguez, M. and Convit, J. A follow-up study of multibacillary Hansen's disease patients treated with multidrug therapy (MDT) or MDT + immunotherapy (IMT). Int. J. Lepr. 65 (1997) 320-327.
18. Sharma, P., Kar, H. K., Misra, R. S., Mukherjee, A., Kaur, H., Mukherjee, R. and Rani, R. Disabilities in multibacillary leprosy following MDT with and without immunotherapy with Mycobacterium w antileprosy vaccine. Int. J. Lepr. 67 (1999) 250-258.
19. Talwar, G. P., Zaheer, S. A., Mukherjee, R., Walia, R., Misra, R. S., Sharma, A. K., Kar, H. K, Mukherjee, A., Parida, S. K., Suresh, N. R., Nair, S. K. and Pandey, R. M. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w , in multibacillary leprosy patients. Vaccine 8 (1990) 121-129.
20. 20. Thuc, N. V., Abel, L., Lap, V. D., Oberti, J. and Lagrange, P. H. Protective effect of BCG against leprosy and its subtypes: a case-control study in southern Vietnam. Int. J. Lepr. 62 (1994) 532-538.
21. Vallishayee, R. S., Gupte, M. D., Anantharaman, D. S. and Nagaraju, B. Post-vaccination sensitization with ICRC vaccine. Indian J. Lepr. 68 (1996)167-174.
22. Waters, M. F. R., Ridley, D. S. and Lucas, S. B. Positive Mitsuda response in long-term treated lepromatous leprosy. Lepr. Rev. 61 (1990) 347-352.
1. D.V.D., National Institute of Immunology, New Delhi 110 067, India.
2. Ph.D., National Institute of Immunology, New Delhi 110 067, India.
3. Ph.D., National Institute of Immunology, New Delhi 110 067, India.
4. Ph.D., National Institute of Immunology, New Delhi 110 067, India.
5. M.D., Department of Dermatology, Venereology and Leprology, Dr. Ram Manohar Lohia Hospital, New Delhi 110 001, India.
6. M.D., Department of Dermatology and Leprology, Safdarjung Hospital, New Delhi 110 029, India.
7. M.D., Institute of Pathology, Indian Council of Medical Research, Safdarjung Hospital Campus, New Delhi 110 029, India.
Reprint requests to Dr. Rajni Rani, Neuroimmunology Division, National Institute of Immunology, New Delhi 110 067, India. FAX: 91-11-616-2125; e-mail: rajni@nii.res.in
Received for publication on 28 December 1998.
Accepted for publication in revised form on 29 June 1999.