- Volume 67 , Number 3
- Page: 308–9
Single-dose treatment for paucibacillary leprosy; feasibility of long-term follow up
To the Editor:
The recommendation by the World Health Organization (WHO) Expert Group (8) that single skin lesion paucibacillary (SSL-PB) leprosy may be treated with a single dose of rifampin 600 mg, ofloxacin 400 mg and minocycline 100 mg (ROM-1) has been received with considerable reservation by clinicians and microbiologists (2-6). In the meantime preliminary short- term experiences of ROM-1 in PB patients with two to three lesions also have been reported (3,5). We have now extended ROM-1 to the whole range of PB patients, i.e., those with one to live lesions.
We believe that any question about the rationale of such an extremely short course of treatment and speculation about the adverse results can be resolved only after long-term observations. Leprosy is characterized by delayed problems in some patients, whatever the form of chemotherapy employed. Such clinical events may be as- cribable to live Mycobacterium leprae or its antigens. It has been reported that a patient developed a nerve abscess 30 years after registration for treatment and 11 years after withdrawal of treatment (1).
The following questions may therefore arise: 1) Can a field program maintain a large number of patients with minimal disease (e.g., SSL-PB) for a sufficiently long period of time? 2) What is the frequency of occurrence and severity of clinical problems encountered? 3) How can such problems be dealt with and managed using field staff? 4) Are the unmanageable? 5) If managed by temporary measures, what is the recurrence rate? and 6) Are the problems within acceptable limits from an epidemiological standpoint and what are the field implications? We have made an attempt to see how many of these questions related to ROM-1 can be answered in a study extending up to 42 months.
Two groups of patients, SSL-PB and paucibacillary with two to five lesions [PB (2-5)], totaling 634 subjects were followed up after a single supervised dose for a period of 42 months in an urban setting. After registration, a spot map of each patient's residence was recorded and volunteers from the community, under supervision of trained workers, made house visits. The clinical events encountered were of the following nature: 1) erythema of existing lesions with or without the appearance of new erythematous lesions; 2) an increase in the existing size of the lesion/with or without appearance of new lesions; and 3) persistence of the existing lesions without any change.
The volunteers were given simple training to deal with any of the above clinical problems and then to report them to the trained workers. Our experience shows that even in a difficult urban setting dealing with delayed clinical events in a large group of patients can be carried out successfully by raising community involvement. The monitoring of the due date and recording of clinical events was done with the help of computers.
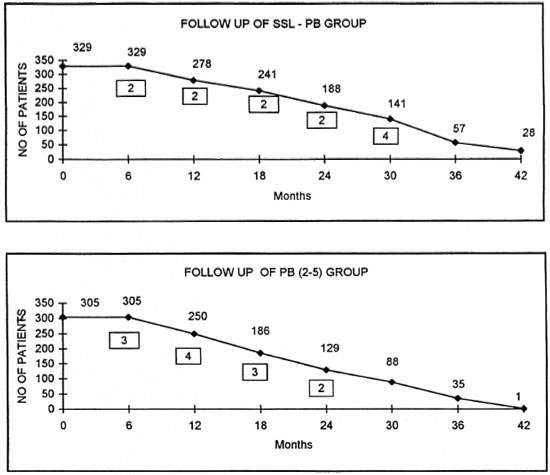
The graphs on page 308 show the follow up of two groups of patients as they were available for examination (not a cohort) and the occurrence of clinical problems. The figures within boxes indicate the number of cases with clinical problems encountered.
The pattern of clinical problems in the two patient groups in relation to the duration of follow up indicates a lack of any correlation between the problems encountered and the chemotherapy interventions adopted. The nature and management of these problems and their field implications are dealt with elsewhere in this issue (4,7). The cause-and-effect relationship is not clearly established by this study. It is also possible that this may be the feature in relation to PB leprosy patients receiving 6 months of WHO/MDT also.
- R. Ganapati
C. R. Revankar
V. V. Pai
S. Kingsley
Bombay Leprosy Project
Vidnyan Bhavan
11, V N Purav Marg
Sion-Chunabhatti
Mumbai 400 022, India
REFERENCES
1. Abrahan, S., Vijaykumaran, P. and Jesudasan, K. Ulnar nerve abscess in a multibacillary patient during post-multidrug therapy surveillance. Lepr. Rev. 68(1997) 333-335.
2. Katoch, V. M. Is there a microbiological rationale for single-dose treatment of leprosy? (Editorial) Lepr. Rev. 69(1998)2-5.
3. Multicentric Trial Group, India. Study of single dose chemotherapy in paucibacillary leprosy patients with two to three lesions. (Abstract) Int. J. Lepr. 66(1998)4A.
4. Pai, V. V., Revankar, C. R., Bui.chand, H. O. and Ganapati, R. Single-dose treatment for paucibacillary leprosy; clinical problems and management. (Letter) Int. J. Lepr. 67(1999)310-312.
5. Pai, V. V., Revankar, C. R., Pai, R. R., Das, M. and Ganapati, R. ROM single dose for PB leprosy with I to 3 lesions. (Abstract) Int. J. Lepr. 66(1998)2A.
6. Ramu, G. Single dose rifampicin, ofloxacin and minocycline (ROM) therapy for single leprosy lesions. (Letter) Indian J. Lepr. 70(1998)78-81.
7. Revankar, C. R., Pai, V. V., Antony Samy, M. S. and Ganapati, R. Single-dose treatment for paucibacillary leprosy; field implications. (Letter) Int. J. Lepr. 67(1999)312-314.
8. WHO Expert Committee on Leprosy. Seventh report. Geneva: World Health Organization, 1998, p. 7. Tech. Rep. Ser. 874.