- Volume 67 , Number 3
- Page: 312–4
Single-dose treatment for paucibacillary leprosy; field implications
To the Editor:
Even though the single-dose treatment of single skin lesion paucibacillary (SSL-PB) leprosy with a single dose of rifampin, ofloxacin and minocycline (ROM-1) is effective and easy to administer, some delayed clinical problems/events, such as the appearance of new lesions, extension and persistence of existing lesions including relapse, have been reported (6). Further observations on such delayed clinical problems in SSL-PB and paucibacillary leprosy patients with two to five lesions [PB (2-5)], their clinical management and feasibility of long-term follow up to identify such events are reported elsewhere in this issue (1,4). More and more such delayed clinical presentations are expected to be encountered by field workers over time and treated and reported as relapses/treatment failure, thus inflating the magnitude of the problem. There is a need to quantify and qualify such events in the field which are to be treated as relapses or treatment failure and to interpret their significance in relation to patients benefitting from ROM-1 in a time-bound public health program like leprosy elimination.
We present here an analysis of 24 such clinical problems after ROM-1 treatment in 329 SSL-PB and 305 PB (2-5) leprosy patients compared with such events already reported as relapses after PB-MDT. These clinical problems were recorded between 6 and 42 months of post-treatment surveillance. The mean period of follow up was 2.1 years in the SSL-PB and 1.8 years in the PB (2-5) leprosy groups. This is a treatment period cohort analysis. The confidence interval (CI) is calculated for all of the values.
Table 1 shows that the rate of occurrence of clinical problems is equal in both groups irrespective of the number of skin lesions. The overall rate is 4% (95% CI 1.8-6.2) or 19 cases (95% CI 16.8-21.2) per 1000 person-years of follow up. Of the 24 cases, 8 remain as delayed clinical problems even after steroids. Of the 8 (1.3%) cases, one from the SSL-PB group was considered as a relapse and retreated with ROM-1 (6). This gave an overall relapse rate of 0.2% (95% CI 0.16-0.56) over 3.5 years or 0.8 relapses (95% CI 0.3-1.3) per 1000 person- years of follow up. The annual rate is 0.06%. If we consider all of these 8 patients as treatment failure/relapse cases, the rate would be 1.3% (95% CI 0.4-2.2) over 3.5 years or 6 cases (95% CI 4.7-7.3) per 1000 person-years of follow up.
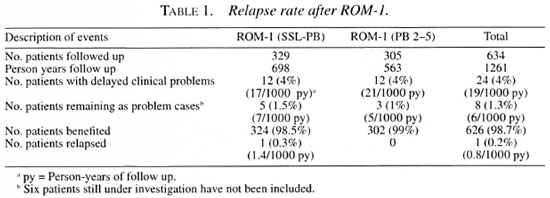
The relapse rate in ROM-1 is less than the already reported relapse rate in PB-MDT patients (Table 2). Another significant finding in this study is that overall 98.7% (95% CI 97.3-99.6) of the patients derived benefit from a single dose (Table 1). This rate is 98.5% (95% CI 97.2-99.8) in SSL-PB and 99% (95% CI 97.9-100) in the PB (2-5) leprosy groups, indicating that the latter group behaved similarly to SSL-PB patients. This is comparable with the rate of WHO-PB-MDT treated patients calculated from previously reported studies.
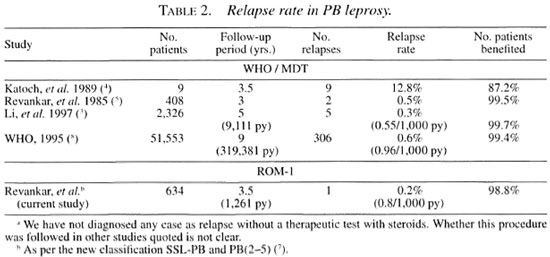
This follow-up study indicated that delayed clinical problems in the form of relapses/treatment failures observed in SSL- PB and PB (2-5) leprosy cases have not been encountered in such an alarming proportion and are within limits manageable by field workers. However, such a low proportion of delayed clinical problems like relapses may assume importance over time.
- C. R. Revankar
V. V. Pai
M. S. Antony Samy
R. Ganapati
Bombay Leprosy Project
Vidnyan Bhavan
11, V N Purav Marg
Sion-Chunabhatti
Mumbai 400 022, India
REFERENCES
1. Ganapati, R., Rkvankar, C. R., Pai, V. V. and Kingskey, S. Single-dose treatment for paucibaeil- lary leprosy; feasibility of long-term follow up. (Letter) Int. J. Lepr. 67(1999)308-309.
2. Katoch, K., Ramanatiian, U., Natrajan, M., Bagga, A. K., Biiatia, A. S., Saxena, R. K. and Ramij, G. Relapse in paucibaeillary patients after treatment with three short-term regimens containing rifampin. Int. J. Lepr. 52(1989)458-464.
3. Li, II. Y., Hu, F. L., Hueng, W. B., Lit:, G. C., Li, J. L. and Yang, Z. M. Risk of relapse in leprosy after lixed-duration multidrug therapy. Int. J. Lepr. 55(1997)238-245.
4. Pai, V. V., Buix iiand, H. O., Revankar, C. R. and Ganapati, R. Single-dose treatment for paueibacil- lary leprosy; clinical problems and management. (Letter) Int. J. Lepr. 67(1999)310-312.
5. Revankar, C. R.. Karjivkak, V. G., Gurav, V. J. and Ganapati, R. Clinical assessment of paucibaeillary leprosy patients under multidrug therapy; three years' follow-up study. Indian J. Lepr. 61(1989)355-359.
6. Rkvankar, C. R., Pai, V. V., Bui c hand, II. O. and Ganapati, R. Delayed clinical problems in single lesion PB leprosy after single dose ROM treatment. (Abstract) Int. J. Lepr. 66(1998)16A.
7. WHO Expert Committee on Leprosy. Seventh report. Geneva: World Health Organization, 1998, p. 7. Tech. Rep. Ser. 874.
8. WHO Leprosy Unit. Risk of relapse in leprosy. Indian J. Lepr. 67(1995)13-26.