- Volume 67 , Number 3
- Page: 279–86
Rapid method for diagnosis of leprosy by measurements of antibodies to the M. leprae 35-kDa protein: comparison with PGL-I antibodies detected by ELISA and "dipstick" methods
ABSTRACT
A new rapid immuno-chromatographic test card for the detection of antibodies to the Mycobacterium leprae 35-kD protein is described. The new assay is compared in the same group of subjects with a direct enzyme ELISA method for 35-kD antibodies and with assays for anti-phenolic glyco- lipid-I (PGL-I) antibodies using a standard ELISA as well as the recently described "dipstick" method. Good concordance was found between the rapid methods and the corresponding ELISA methods. The detection of untreated paucibacillary leprosy by the 35-kD test card was 59% compared with 27% for the PGL-I dipstick; however, the specificity for the 35-kD test card was 90% compared with 100% for the PGL-1 dipstick in an endemic population. The potential application of these new, rapid serologic methods for the diagnosis of leprosy under field conditions is discussed.RÉSUMÉ
Un nouveau test rapide immuno-chromatogra- phique sur cartes, pour la détection d'anticorps dirigés contre la protéine de 35 kDa de Mycobacterium leprae est présenté. Cette nouvelle méthode est comparée chez, le même groupe de sujets avec un test direct de type ELISA mesurant les anticorps contre la protéine de 35 kDa, et des tests mesurant les anticorps contre le glycolipide phénolique de type 1 (PGL-1) utilisant une méthode ELISA standard, ainsi que la méthode récemment décrite dite de la bandelette (dispstick). Une bonne concordance fut trouvée entre les méthodes rapides et les méthodes ELISA correspondantes. La détection des cas non-traités de lèpre paucibacillaire par le test sur carton de la protéine de 35 kDa était de 59% comparé à 27% pour le PGL-1 par la bandelette; cependant, la spécificité du test sur carton de la protéine de 35 kDa était de 90% comparé à 100% pour le PGL-1 par la bandelette dans une population endémique. Les applications potentielles de ces nouvelles méthodes sérologiques rapides pour le diagnostic de la lèpre sur le terrain sont discutées.RESUMEN
Se describe una nueva y rápida prueba immunocro- matográfica en tarjeta para la detección de anticuerpos contra la proteína de 35 kD de Mycobacterium leprae. El ensayo se compara en el mismo grupo de sujetos con una prueba de ELISA para el mismo antígeno y con dos ensayos para el glicolipido fenólico-I (PGL-I): un ELISA y la recienteniente descrita prueba de la tira reactiva. Se encontro una buena concordância entre las pruebas rápidas y los ELISAs correspondientes. La detección de pacientes paucibacilares usando la prueba de la tarjeta para la proteína de 35 kD Cue del 59% comparada con el 27% para la prueba de la tira reactiva para el PGL-I; sin embargo, la especificidad para la prueba de la tarjeta (35 kD) fue del 90% comparada con el 100% para la tira reactiva (PGL-I) en una población endêmica. Se discute la aplicación potencial de estos nuevos métodos serológicos rápidos en el diagnóstico de la lepra bajo condiciones de campo.The measurement of antileprosy antibodies has been utilized as a means of detecting leprosy exposure (1-3), classifying patients with clinical disease (7-10), monitoring the response to chemotherapy (8), and defining patients at increased risk of type 1 reactions (10,11).
The 35-kD protein of Mycobacterium leprae is a major target for the antibody response in lepromatous leprosy (8). In the past, the only assay to detect such antibodies relied on the inhibition of the binding of a labelled monoclonal antibody to the protein present in sonicates of the leprosy bacillus (12). The 35-kD protein has recently been cloned, sequenced, and expressed as a recombinant protein in the fast-growing mycobacteria M. smegmatis . This protein retains the conformational epitope recognized by leprosy sera (14). Recent studies have shown that an enzyme-linked immunosorbent assay (ELISA) using the recombinant protein has an equivalent sensitivity and specificity to that of the monoclonal inhibition ELISA (13).
Phenolic glycolipid-I (PGL-I) is an abundant cell-surface glycolipid of M. leprae with a unique terminal trisaccharide (6) which is the target for a strong IgM antibody response in lepromatous leprosy patients (4).
The major drawback in the application of serology to leprosy control programs has been the need to establish a laboratory capable of performing the ELISAs. The use of dried blood samples has allowed central laboratories to collect samples for serology from widely distributed clinics. However, a rapid bedside test would clearly be preferable. The need for new leprosy diagnostics will increase as a decline in the prevalence of clinical leprosy is accompanied by a decline in the availability of other diagnostic tests, such as the slit-skin smear.
In this paper we report for the first time the use of a rapid antibody detection kit using the M. leprae 35-kD protein and compare this assay procedure with the standard ELISA. In addition, we compare the sensitivity and specificity for leprosy disease with that obtained using the anti-PGL-I assay as measured both by conventional ELISA and by the new "dipstick" method (2).
MATERIALS AND METHODS
Patients. A total of 174 patients and healthy controls were tested. These included 111 males and 63 females with an age range of 9 to 81 years. The study group was composed of 10 healthy Nepali persons with no known contact with leprosy or tuberculosis; 30 Nepali tuberculosis patients (16 had pulmonary disease, 11 of whom were smear positive, and 14 had nonpul- monary disease); 47 healthy contacts of leprosy patients (38 household and 9 non- household contacts), and 87 leprosy patients [5 tuberculoid (TT), 31 borderline tuberculoid (BT), 2 borderline (BB), 25 borderline lepromatous (BL), 18 leproma- tous (LL) and 6 primary neuritic (PN)]. Of the leprosy patients, 50 had had no previous antileprosy treatment and 37 had had treatment of varying lengths.
Assay 1. IgM anti-PGL-I antibodies were measured by an ELISA as previously described (7). Briefly, wells of a microliter tray (Dynatech, Chantilly, Virginia, U.S.A.) were coated with 100 µl of disaccharide- bovine serum albumin (d-BSA, supplied by IMMYC, World Health Organization), 250 ng/ml in 0.05 M carbonate buffer, pH 9.6, blocked with 200 µl 1% w/v BSA and incubated with patient sera diluted 1:300 in 10% normal goat serum (NGS) in phosphate buffered saline (PBS). After washing with PBS 0.05% Tween 20 (PBST), 100 µl goat anti-human IgM-peroxidasc conjugate (Cappel Laboratories, West Chester, Pennsylvania, U.S.A.) diluted 1:4000 in 10% NGS/PBS was added before incubation with 100 µl 0.4 g/L o -phenylenediamine (OPD; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) in 0.05 M citrate phosphate buffer, pH 5.0, containing 0.006% hydrogen peroxide. The reaction was stopped after 10 min with 100 µl of 2.5 M sulfuric acid, and the plates were read at 492 nm in a MRX microplate reader (Dynatech). Sera with an absorbance at 492 nm of greater than or equal to 0.2 (which is the mean plus 3 standard deviations of 50 Nepali healthy control sera) were considered positive.
Assay 2. IgG anti-35-kD antibodies were measured as previously described (n). Briefly, microtiter trays were coated with 100 µl of 10 pg/ml of the purified recombinant 35-kD protein in carbonate buffer, pH 9.6, blocked with 200 µl 3% BSA and then incubated with patient sera diluted 1 in 100 in 1% BSA. After washing with PBST, 100 ml of anti-human IgG-pcroxidase conjugate, diluted 1 in 1000 in 1% BSA was added before incubation with OPD substrate for 20 min. Samples with an absorbance greater than 0.42, which was the mean of 50 Nepali healthy control sera plus 2 standard deviations, were considered positive.
Assay 3. Anti-35-kD cards were supplied by Amrad ICT Diagnostics, Brook- vale, NSW, Australia (Fig. 1A). This consisted of a unique cardboard folder (5). The right-hand face contained a nitrocellulose (NC) strip (6 mm x 22 mm) on which the 35-kD antigen was applied (T). A control, namely, polyclonal anti-human Ig antisera (C), was applied as a positive control. At the base and top of the NC strip were thin absorbent pads (1 and 2). The left-hand face of the device had a window cut into it (5). A conjugate pad (4) was situated above the window on which 5 µl of goat anti-human IgG conjugate linked to colloidal gold was dried. There was a thick absorbent pad below the window. Two drops of buffer reagent were added to the lower pad (1) followed by an application of 30 µl of serum to the upper pad above the NC strip (2). Scrum was allowed to diffuse down the NC to the marked line (L), at which point one drop of buffer was added to the transfer pad (3). At this point the folder was closed with an adhesive strip. If a patient has antibodies to the 35-kD protein, a red line will start to appear within 5 min of closing the apparatus. The final result of the test was read after 15 min.
Assay 4. Dipstick method. The detection of anti-PGL-I antibodies by the dipstick method was performed as previously described (2). Briefly, dipsticks had the synthetic antigen disaccharide-BSA conjugate immobilized on a NC strip. An internal control was provided by anti-human IgM antibodies immobilized in a lower band on the same NC strip. Scrum was diluted 1:50 in detection reagent which was composed of a monoclonal anti-human IgM conjugated to palinal red, a colloidal dye. Detection strips were placed directly into the diluted samples after pre-wetting, incubated for 3 hr at room temperature, then rinsed, air dried and read. A reddish-stained antigen band indicated a positive reaction. Stainings of varying intensities were scored as positive: the absence of color was scored as negative.
Statistical analysis. The differences between groups in the proportion of subjects seropositive were tested by means of the chi-squared test. The degree of concordance between assays measuring the same antibodies by means of different assays was calculated by determining the kappa values which express agreement between methods beyond that due to chance. A kappa value of 1.0 reflects perfect agreement.
RESULTS
Rapid methods. All assays were performed by staff with considerable serological experience. Under these conditions, the 35-kD test card was found to be easy to use and generally unequivocal in the results obtained (Fig. 1B). Similarly, the PGL-I dipstick was easy to use, although there were more handling steps (pre-wetting, dispensing detection reagent, diluting serum and rinsing dipstick), the time to result was considerably longer (3 hr compared with 15 min), and some of the sera gave weak color responses.
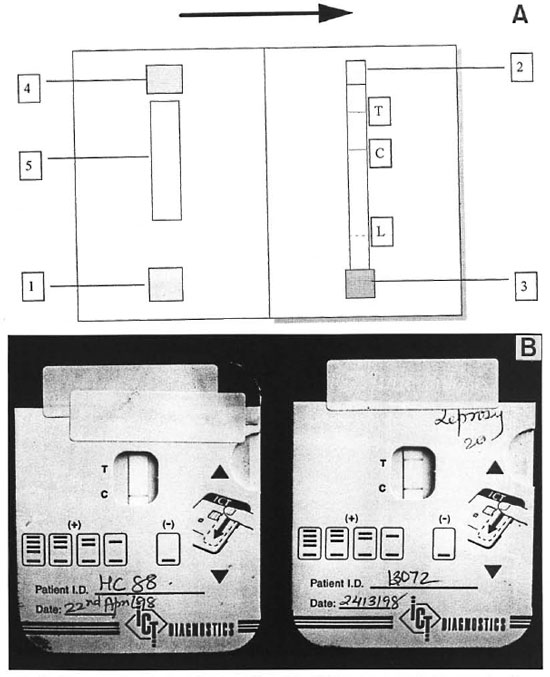
Fig. 1. Diagrammatic and pictorial representation of the 35-kD test card used in this study. A = Diagramshowing details of the opened device. 1. absorbent pad, 2. transfer pad, 3. serum pad, 4. conjugate pad, 5. win-dow, T = test antigen line on nitrocellulose strip, C = control line, L = limit line to which serum is allowed to run. B = Two 35-kD test cards: one from a positive sample (LL patient, ELISA A492 = 0.509) and one from a control subject (A492 = 0.1). The internal control band is positive in both.
Comparison between ELISAs and rapid methods. Table 1 shows the comparison between the 35-kD test card and the direct ELISA and between the PGL-I dipstick and the ELISA. A higher degree of concordance was noted between the PGL-I dipstick and the PGL-I ELISA (93.7%, p <0.001) than between the 35-kD test card and the corresponding ELISA (75.3%, p <0.001). The adjusted kappa values for both sets of data were 0.81 for the PGL-I assays and 0.47 for the 35-kD assays.
The relationships between the ELISAs and the rapid methods is shown in Figure 2. In panel A, the distribution of ELISA results in absorbance units for the 35-kD test card positive and negative groups are shown: 20 subjects (2 leprosy contacts, 3 TB patients and 18 leprosy patients) were positive for the test card but negative in the anti-35-kD ELISA (mean A492 = 0.311, S.D. = 0.07), while a further 23 subjects (8 contacts, 1 TB patient and 11 leprosy patients) were test card negative but positive by the anti-35 kD ELISA (mean A492 = 0.566, S.D. = 0.168).
In panel B of Figure 2, the distribution of ELISA results in absorbance units for the PGL-I dipstick-positive and -negative groups are shown: 4 leprosy patients were positive in the dipstick assay while negative by anti-PGL-I ELISA (mean A492 = 0.077, S.D. = 0.023) and 7 subjects (6 leprosy patients and 1 TB patient) were dipstick negative while ELISA positive (mean A492 = 0.287, S.D. = 0.083).
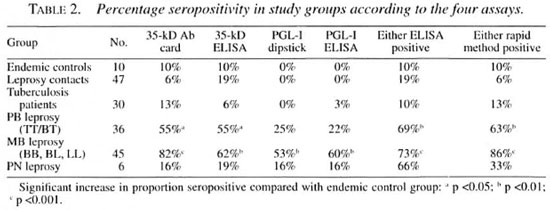
Serological test results in different study groups. Table 2 shows the proportion positive for each group for each of the four assays. There were no significant differences in the proportions positive in each group as measured by ELISA compared with the rapid methods.
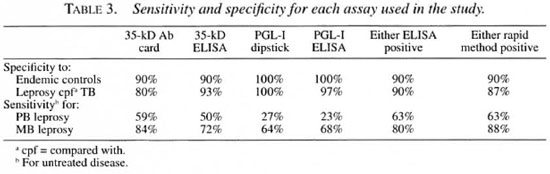
The PGL-I assays showed high specificity and lower sensitivity for detection of untreated leprosy compared with the 35-kD assays which had lower specificity but higher sensitivity (Table 3). There were no significant differences in the detection of untreated leprosy of any classification between rapid and standard ELISAs for either of the two antibody types. A combination of both assays, whether ELISA or rapid, led to a significant improvement in sensitivity for detecting paucibacillary (PB) leprosy with a small decrease in specificity.
DISCUSSION
This study establishes a new rapid method for detecting antileprosy antibodies by measuring antibodies to the M. leprae 35-kD protein by an immuno-chromato- graphic method. The concordance between the rapid method and the standard direct ELISA using the same antigen is high, although the concordance is not yet satisfactory. The sensitivity for detecting untreated PB leprosy is significantly better than that of the anti-PGL-I assays, but there is a lower specificity.
The reasons for the lower specificity of the anti-35-kD assays for leprosy are probably the result of the recombinant expression system used to produce the M. leprae 35-kD protein. As previously reported, the expression system for this protein in the expression of this protein in standard Esch- rapidly growing mycobacterium M. smeg- erichia coli expression systems failed to mat is, which yielded a "native'Mike protein produce a soluble protein and it failed to with the correct folding to give the antibody produce protein with the major antibody epitope. The protein purified by mono- epitope for the monoclonal ML04, which is clonal affinity chromatography from soni- also the dominant epitope for binding by cates of M. smegmatis , however, contains a leprosy sera (14). This led us to develop an variable amount of contaminating mycobactcrial host-cell proteins to which both endemic healthy subjects as well as patients with tuberculosis may have antibodies. The amount of host-ccll contamination varies from batch to batch, and this variation may explain the relatively high discordance between the 35-kD test card and the ELISA method. New expression systems in M. smegmatis with M. smegmatis -specific "tags" to allow better purification from host proteins will reduce the nonspecific antibody binding and improve the specificity of the assay.
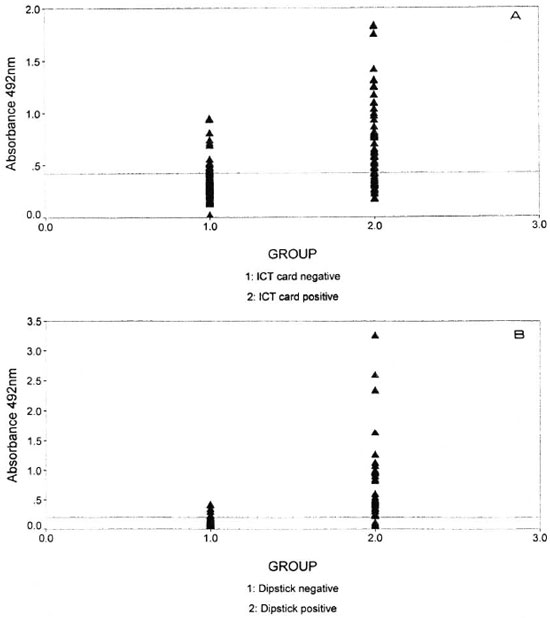
Fig. 2. Graphic representation of the ELISA and rapid results with 174 sera. Panel A shows the disi ibutionof ELISA results for the 35-kD test card-positive and -negative groups. Panel B shows the distribution of ELISA results for the PGL-I dipstick-positive and -negative groups. The horizontal line in each panei indicates the cut-off value for the ELISA.
The rapid method for detecting antibodies to PGL-I shows a high concordance to the ELISA method (2). The PGL-I antibodies, however, are detectable in a loWer proportion of PB leprosy patients than the anti- 35-kD antibodies, whether measured by the methods shown here or by the monoclonal inhibition assay (7). The combination of the two assays significantly improves the detection of untreated PB leprosy. Further, the rapid assay methods could in the future be combined to allow the simultaneous detection of both anti-35-kD and anti-PGL-I antibodies in the same sample. In addition, both assays are adaptable to the use of whole blood in the place of serum, further simplifying the procedure and making the field application of the test(s) more feasible.
As registered leprosy prevalence continues to dccline, skills and experience necessary for the diagnosis of the disease will become more rare. In countries with a low leprosy prevalence, new technologies like the ones described in this paper will be needed to allow the timely diagnosis of leprosy.
Acknowledgment. This work was supported by The Leprosy Mission International. The authors would like to thank the staff and patients of Anandaban Hospital for their cooperation in this study. We thank Dr. Paul Klatser and Dr. Saillira Buhrer, Department of Biomedical Science, Royal Tropical Institute, Amsterdam, The Netherlands, for supplying the PGL-I antibody dipsticks for this study and for their valuable suggestions on the manuscript. This work was presented as a poster at the International Leprosy Congress, Beijing, China September 7-12, 1998 (Abstract no: IM59).
REFERENCES
1. Baumgart, K. W., Britton, W. J., Muluns, R. J., Basten, A. and Barnetson, R. St.C. Subclinical infection with Mycobacterium leprae - a problem for leprosy control strategies. Trans. R. Soc. Trop. Med. Hyg. 87 (1993) 412-415.
2. Buhrer, S. S., Smits, H. L., Gussenhoven, G. C., van Ingen, C. W. and Klatser, P. R. A simple dipstick assay for the detection of antibodies to phenolic glycolipid-I of Mycobacterium leprae . Am. J. Trop. Med. Hyg. 58 (1998) 133-136.
3. Cho, S.-N., Kim, S. H., Cellona, R. V., Chan, G. P., Fajardo, T. T., Walsh, G. P. and Kim, J. D. Prevalence of IgM antibodies to phenolic glyco- lipid-I among household contacts and controls in Korea and The Philippines. Lepr. Rev. 63 (1992) 12-20.
4. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Gei.ber, P. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41 (1983) 1077-1083.
5. Cole, R. A., Lu, H. M., Shi, Y. Z., De-Hua, T. and Zhou, A. T. Clinical evaluation of a rapid immuno-chromatographic assay based on the 38- kDa antigen of Mycobacterium tuberculosis on patients with pulmonary tuberculosis in China. Tuberc. Lung Dis. 77 (1996) 363-368.
6. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacterid. 147 (1981) 728-735.
7. Roche, P. W., Britton, W. J., Failbus, S. S., Lundwig, H., Theuvenet, W. J. and Adiga, R. B. Heterogeneity of serological responses in paucibacil- lary leprosy: differential responses to protein and carbohydrate antigens and correlation with clinical parameters. Int. J. Lepr. 58 (1990) 319-327.
8. Roche, P. W., Britton, W. J., Failbus, S. S., Williams, D., Pradhan, H. M. and Theuvenet, W. J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. 58 (1990) 480-490.
9. Roche, P. W., Failbus, S. S., Neupane, K. D., Theuvenet, W. J. and Britton, W. J. Serological monitoring of the response to chemotherapy in leprosy patients. Int. J. Lepr. 61 (1993) 35-43.
10. Roche, P. W., LeMaster, J. W. and Butun, C. R. Risk factors for type 1 reactions in leprosy. Int. J. Lepr. 65 (1997) 450-455.
11. Roche, P. W., Theuvenet, W. J. and Britton, W. J. Risk factors for type-1 reactions in borderline leprosy patients. Lancet 338 (1991) 654-657.
12. SlNHA, S., Si:nguita, U., Ramu, G. and Ivanyi, J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int. J. Lepr. 53 (1985) 33-38.
13. Triccas, J. A., Rochk, P. W. and Bkitton, W. J. Specific serological detection of leprosy with a recombinant Mycobacterium leprae protein purified from a rapidly growing mycobacterial host. J. Clin. Microbiol. 36 (1998) 2363-2365.
14. Triccas, J. A., Rochi;, P. W., Winter, N., Feng, C. G., Buti.in, C. R. and Bkitton, W. J. A 35- kDa protein is a major target of the human immune response to Mycobacterium leprae . Infect. Immun. 64 (1996) 5171-5177.
1. Ph.D., Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
2. M.Sc., Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
3. Ph.D., Centenary Institute of Cancer Medicine and Cell Biology, Sydney, Australia.
4. Ph.D., Amrad ICT Diagnostics, P.O. Box 228, Brookvale, New South Wales, Australia.
Reprint requests to Paul W. Roche, Ph.D. at the above address or FAX: 977-1-290-538; e-mail: roche@umn.mos.com.np
Received for publication on 28 December 1998.
Accepted for publication in revised form on 20 may 1999.