- Volume 67 , Number 3
- Page: 287–91
Serum IL-1ra is elevated in lepromatous leprosy patients
ABSTRACT
Patterns of production of specific cytokines are accepted as standards for T-lymphocyte subsets in diseases caused by intracellular parasites. These lymphocyte subsets (Th1 and Th2) have been associated with the different poles of the leprosy spectrum. Lepromatous leprosy (LL) onset correlates with cytokines produced by Th2 cells on the grounds of the patient's poor cellular immune response, i.e., interleukin 2 (IL-2) and gamma interferon (IFN-γ) deficiency. On the other hand, tuberculoid leprosy (TL) has been associated with a Th1 response. Moreover, pro-inflammatory cytokines like IL-1β and tumor necrosis factor-alpha (TNF-α) play a major role in chronic inflammatory pathologies being IL- 1ra and TNF-α soluble receptors, natural counterbalancing inhibitors. In light of this background, we decided to measure serum levels of IL-1β, IL-1ra, TNF-α and IL-6 in LL and TL patients, and we also studied the production in vitro of Th1 (IFN-γ, IL-2), Th2 (IL-4, IL-10) and TNF-α cytokines. Our data showed that IL-1ra is highly elevated in sera f rom LL patients; there were no differences in Th2 cytokine levels and there were diminished levels in Th1 cytokines.<RÉSUMÉ
Les profils de production dy cytokines spécifiques sont des mesures maintenant acceptées d'évaluation des sous-populations de lymphocytes T présentes dans les maladies causées par des parasites intracellulaires. Ces sous-populations de lymphocytes T (Th1 et Th2) ont été associées aux différents pôles du spectre de la lèpre. La laepre lépromateuse (LL) est associée à la sécrétion de cytokines par des lymphocytes de type Th2 et ets caractérisée par une faible réponse immunitaire à médiation cellulaire du patient, c'est-à-dire une déficience de production d'interleukine 2 (IL-2) et d'interféron gamma (IFN-γ). La laepre tuberculoïde (TL), en revanche, a ;aaté associée a une réponse de type Th1. De plus, les cytokines pro-inllammatoires comme l'IL-1β et le facteur de nécrose des tumeurs- alpha (TNF-α) jouent un rôle majeur dans les états pathologiques inflammatories chroniques avec, comme inhibiteurs et rétrocontrôles négatifs, l'IL-1ra et les récepteurs solubles de TNF-α. Au vu de ces connaissances, nous avons décidé de mesurer les niveaux sériques de l'IL-β, de l'IL-1ra, du TNF-α et de l'IL- 6 chez des patients LL et TL, ainsi que la production in vitro des cytokines de type Th1 (IFN-γ, IL-2), de type Th2 (IL-4, IL-10) et de TNF-α. Nos résultats indiquent que IL-1ra est présente en grande quantité dans le sérum des patients LL. Il n'y avait pas de différence de niveaux de sécrétion des cytokines de type Th2 et les niveaux de sécrétion des cytokines de type Th1 étaient diminués chez les patients LL>RESUMEN
Se reconocen patrones específicos de producción de citocinas por Ias subpoblaciones de linfocitos T en enfermedades causadas pro parásitos intracelulares. Estas subpoblaciones de linfocitos (Th1 y Th2) se han encontrado asociadas con los diferentes polos del espectro de la lepra. La lepra lepromatosa correlaciona con las citocinas producidas por Ias células Th2 (inter- leucina 2 e interferon gamma) y con una pobre res- puesta immune celular. Por el contrario, la lepra tuberculoide se ha asociado con una respuesta Th1, Además, Ias citocinas pro-infiamatorias como la IL-1β y el factor de necrosis tumoral alfa (TNF-α) juegan un papel importante en las patologias inflamatorias crônicas, siendo el receptor soluble IL-1ra y el receptor para TNF-α, las moléculas reguladoras naturales. Sobre esta base decidimos medir los niveles en el suero de IL-1β, IL-1ra, TNF-α e IL-6 en pacientes LL y TL, y la production in vitro de las citocinas Th1 (IFN-γ, IL-2), Th2 (IL-4, IL-10) y TNF-α. Nuestros datos mostraron que IL-1ra esta muy elevado en el suero de los pacientes LL; no hubieron diferencias en los niveles de citocinas Th2 aunque los niveles de citocinas Th1 estuvieron disminuidos.T lymphocytes are key elements in mediating both cellular and humoral immune responses through cytokine production against infections. Two functional subsets of CD4+ lymphocytes have been characterized based on the production of specific cytokines. Thus, in animal models, Th1 cells produce interleukin 2 (IL-2) and gamma interferon (IFN-γ) preferentially driving the cellular immune response; Th2 cells drive the humoral immune response by secreting IL-4, IL-5 and IL-10. Likewise, feedback among this network of cytokines has been observed. Thus, IFN-γ inhibits Th2 cell function and IL-10 and IL-4 can block Th1 action. These characteristic patterns have been clearly demonstrated in murine experimental models associated with either cellular protection or immunopathological processes. In humans, a clear-cut pattern of these cellular subsets remains to be established mainly due to the fact that healthy individuals present a highly combined pattern of cytokines. Nonetheless, studies in recent years have shown some evidence of polarized subsets of CD4+ T cells in patients with infectious diseases and allergies (9,14,16).
Immunological and clinical manifestations in leprosy patients represent a good model for use in discriminating among patterns of response mounted by different individuals against the same pathogen ( Mycobacterium leprae ). Tuberculoid leprosy (TL) patients develop a strong delayed hypersensitivity response toward M. leprae antigens, as well as a poor antibody-mediated response, resembling a Th1 type response (16,17). On the other hand, lepromatous leprosy (LL) patients have a defective cellular immune system; they do not mount a response against M. leprae antigens, and they display a poor delayed hypersensitivity response and have abundant in vivo antibody production. Moreover, T lymphocytes from LL patients do not proliferate and produce low IL-2 and IFN-γ levels against nonspecific polyclonal stimuli (PHA, ConA, anti-CD3) evoking Th2 type cell functions (4-8,10-12).
Knowledge of the active role of pro-inflammatory and antiinflammatory cytokines in the development of leprosy is lacking. Therefore, in this communication we have tried to elucidate the patterns of specific cytokines in leprosy patients. We found that elevated serum levels of interleukin 1 receptor antagonist (IL-1ra) is a major feature in LL patients.
MATERIALS AND METHODS
Study subjects. Eighteen patients (nine females, nine males; ages 20 to 65) from the Dermatologic Institute, Guadalajara, Jalisco, Mexico, were studied. Thirteen patients were classified as having polar lepromatous leprosy (LL) and six as having tuberculoid (TT) leprosy according to the Ri- dley-Jopling classification (13). The LL patients presented positive bacilloscopy, and all of them received treatment proposed by the World Health Organization (WHO) consisting of dapsone, clofazimine and rifampin (WHO/MDT) (19).
The control group consisted of 10 unrelated healthy subjects sex- and age-matched with the patient group.
Samples. Blood was obtained by venipuncture to establish T lymphocyte cultures and to measure the production in vitro of IFN-γ, 1L-2, 1L-4, IL-10 and tumor necrosis factor-alpha (TNF-α) cytokines. An aliquot of the same blood was used to obtain sera for further evaluation of scrum cytokines IL-1β, IL-1ra, TNF-α and IL-6.
Supernatants of lymphocytes stimulated with phytohemagglutinin (PHA). Mononuclear cells were separated over Histopaque-1077 (Sigma Chemical Co., St. Louis, Missouri, U.S.A.), washed tw-ice in Hank's balanced salt solution (HBSS), and suspended in RPMI 1640 medium (Gibco, Grand Island, New York, U.S.A.) supplemented with 2.5% heat-inactivated fetal calf serum (FCS). Cells were adjusted to 1 x 106 cells/ml and activated with 10 µg PHA/ml (5-17). The cell cultures were incubated for 48 hr at 37ºC in a humidified atmosphere of 95% air and 5% CO2. Cell-free supernatants were collected and appropriately diluted to evaluate IFN-γ, IL-2, IL-4, IL-10 and TNF-α concentration by enzyme linked immunosorbent assays (ELISA) as described by the manufacturer (R&D Systems, Minneapolis, Minnesota, U.S.A.).
Serum cytokine measurements. The Quantikine human IL-1β, IL-1ra, TNF-α and IL-6 immunoassay (R&D Systems) were used for the quantitative determination of human pro-inflammatory cytokines present in sera collected from LL patients, TL patients and controls. Appropriate standard curves were used.
RESULTS
Secretion of pro- and antiinflammatory cytokines by lymphocytes is an important feature of several chronic inflammatory diseases. Therefore, we measured the production of IL-4, IL-10 and TNF-α by mononuclear cells from the LL patients and healthy controls. Table 1 shows that control cells stimulated with PHA did not secrete into the culture media different amounts of IL- 10 and IL-4 as compared with the leproma- tous leprosy patients. It is noteworthy that IL-4 levels were undetectable in the cellfree supernatants from cither group.
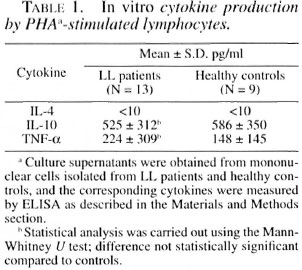
Table 2 shows Th1 cytokine (IL-2, IFN-γ) concentrations in the supernatants of mononuclear cells from LL patients and healthy controls obtained after stimulation with PHA. LL patients produced significantly less IL-2 and IFN-γ than the healthy controls.
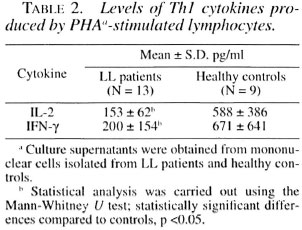
Table 3 shows the in vivo levels of IL-1β, IL-6, TNF-α, and IL-1ra in sera from tuberculoid leprosy patients, lepromatous leprosy patients and healthy controls. The controls showed no detectable IL-6 levels, but slight increases were noticed in sera of TL and LL patients. IL-1p serum levels in LL patients were high as compared with the control group although the difference was not statistically significant. On the other hand, TL patients showed no differences in IL-1β when compared to healthy controls.
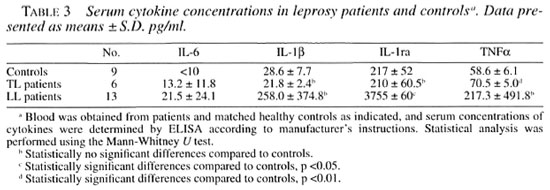
Regarding TNF-α, the serum concentration in TL patients was significantly higher compared with the healthy controls. Even though LL patients had a tendency toward higher levels of TNF-α, there was no statistically significant difference when the TNF-α serum levels were compared to the healthy controls.
Lastly, our most remarkable finding was the 15-fold increase of IL-1ra in sera from LL patients as compared with the healthy controls. The IL-1ra serum concentration in TL patients was no different than that of the control group.
DISCUSSION
The clinical and immunological manifestations in leprosy represent a well-defined system of variable cellular immune responses against the same antigen. On one hand, TL patients develop a good, delayed immune response against M. leprae , effectively controlling the bacterial growth and spreading of the disease. On the other hand, LL patients do not respond specifically against M. leprae antigens. The immune response is poor, thus presenting a multibacil- lary disease with spreading. The Th1 cells response plays a major role in the developing and the control of several infections, while Th2 cytokines (IL-4, IL-5 and IL-10) have been associated with progression of disease (9,16).
Based on results obtained by several groups of researchers, the tuberculoid pole of the leprosy spectrum has been associated with a Th1-type response, while the lepro- matous pole clearly correlates with Th2 response. This was due to the fact that LL patients produce very low concentrations of IL-2 and IFN-γ and high concentrations of IL-4 and IL-10 when compared to healthy individuals (5,7,8,11). Nevertheless, other investigators point out that the pattern of cytokines seems not to correlate with the clinical stage of leprosy patients (4).
In this work we have evaluated supernatant concentrations of Th1 and Th2 cytokines in cultures of LL patients' mononuclear cells, and we found no difference in the Th2 responses between LL patients and healthy individuals. Regarding Th1 cytokine levels, our data presented here confirm and extend previous observations concerning IL-2 and IFN-γ production by lymphocytes (6). Thus, lymphocytes from LL patients produced far less amounts of IL-2 and IFN-γ as compared to healthy subjects (5,11,12). On the other hand, data obtained from the measurement of pro-inflammatory cytokines demonstrated that both TL and LL patients had higher IL-6 serum concentrations compared with controls was expected given the characteristics of this disease.
Our results concerning IL-1ra serum concentrations are important. IL-1ra is a natural inhibitor of cytokine IL-1 (18). A clear-cut relationship among upregulation of IL-1ra and inflammatory events has been demonstrated in HIV infection, rheumatoid arthritis, glomerulonephritis and septic shock (1). In keeping with this, murine experimental models induced by infection with M. avium have shed light on the role of IL-1 ra. Thus, IL-1 ra promotes increased M. avium growth in the infected animals (1). High IL-1ra levels in granulomatous lesions of patients with tuberculosis and sarcoidosis have also been demonstrated (2). Consistent with these data, TNF-α is also an important inducer of IL-1ra production by cells in granulomatous diseases (15). In addition, Apostopulos, et al . (1) have shown that where IL-1 ra is exogenously added, it inhibits proliferation of PHA-stimulated thymocytes.
Taken together, these findings arc consistent with our previous results in which we found that lymphocytes from LL patients clearly displayed an inability to proliferate in vitro before stimulus given by M. leprae antigens and PHA. In this study we have found a dramatic increase of IL-1ra in the sera of LL patients. The interrelationship among these facts deserves further consideration, and the mechanisms involved are currently being investigated in our laboratory.
Acknowledgment. We are indebted to Ing. Rogelio Troyo for his invaluable help in statistical analyses. This study was supported by a grant from Conacyt 1485P-M.
REFERENCES
1. Apostopulos, J., Ross, S., Davenport, P., Mat- sukawa. A., Yoshinaga, M. and Tipping, P. G. Interleukin-1 receptor antagonist: characterization of its gene expression in rabbit tissues and large- scale expression in eucaryotic cells using a bac- ulovirus expression system. J. Immunol. Meth. 199(1996)27-35.
2. Chensue, S. W., Warmington, K. S., Berger, A. E. and Tracey, D. E. Immunohistochemical demonstration of interleukin-1 receptor antagonist protein and interleukin-1 in human lyphois tissue and granulomas. Am. J. Pathol. 140(1992)269-275.
3. Denis, M. and Ghadirian, E. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect. Immun. 62(1994)457-461.
4. Howe, R. C., Wondimu, A., Demisee, A. and Frommel, D. Functional heterogeneity among CD4+ T-cell clones from blood and skin lesions of leprosy patients; identification of T-cell clones distinct from ThO, Th1 and Th2. Immunology 84(1995)585-594.
5. Isi.as-Rodriguez, A. E., Morales-Ortiz, R., Fai utis-Morris, M., Gonzai.ez-Mendoza, A. and Ortiz-Ortiz, L. Deficiency in the biosynthesis of interleukin-2 (IL-2) and functional presence of the IL-2 receptor in lepromatous leprosy. (Letter) Int. J. Lepr. 55(1987)566-569.
6. Kreuzer, K. A., Davi;r, J. M., Rockstroh, J. K., Sauerbruch, T. and Spengler, U. The IL-1 system in HIV infection: peripheral concentrations of IL-1beta. IL-1 receptor antagonist and soluble IL-1 receptor type II. Clin. Exp. Immunol. 109(1997)54-58.
7. Misra, N., Selvakumar, M., Singh, S., Biiarad- waj, M., Ramesh, V., Misra, R. S. and Natii, I. Monocyte derived IL-10 and PGE2 are associated with the absence of Th1 cells and in vitro T cell suppression in lepromatous leprosy. Immunol. Lett. 48(1995)123-128.
8. Modi.in, R. L. Th1-Th2 paradigm: insights from leprosy. J. Invest. Dermatol. 102(1994)828-832.
9. Mosmann, T. R. and Coffman, R. L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann. Rev. Immunol. 7(1989)145-173.
10. Mutis, T., Kraakman, E. M., Cornelisse, Y. E., Haanen, J. B. A. G., Spits, H., de Vries, R. R. P. and Ottenhoff, T. H. M. Analysis of cytokine production by Mycobacterium-reactive T cells. J. Immunol. 150(1993)4641-4651.
11. Nogueira, N., Kaplan, G., Levy, E., Sarno, E. N., Kushner, P., Granelli-Piperno, A., Vierira, L., Colomer Gould, V., Levis, W., Steinman, R., Yip, Y. K. and Coiin, Z. A. Defective y interferon production in leprosy; reversal with antigen and in- terleukin 2. J. Exp. Med. 158(1983)2165-2170.
12. Ochoa, M. T., Valderrama, L., Ochoa, A., Zea, A. Excobar, C. E., Moreno, L. H. and Falabella, R. Lepromatous and tuberculoid leprosy: clinical presentation and cytokine responses. Int. J. Dermatol. 35(1996)786-790.
13. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34(1966)255-273.
14. Romagnani, S. Human Th1 and Th2 subsets: doubt no more. Immunol. Today 12(1991)256-257.
15. Ruth, J. H., Bienkowski, M., Warmington, K. S., Lincoln, P. M., Kunkel, S. L. and Chensue, S. W. IL-1 receptor antagonist (IL-1 ra) expression, function and cytokine-mediated regulation during mycobacterial and schistosomal antigen-elicited granuloma formation. J. Immunol. 156(1996)2503-2509.
16. Shearer, G. M. Th1/Th2 changes in aging. Mech. Ageing Develop. 94(1997)1-5.
17. Surcel, H. M., Troye-Bi.omberg, M., Paulie, S., Andersson, G., Moreno, C., Pasvol, G. and Ivanyi, J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology 81(1994)171-176.
18. Suzuki, K., Fukutomi, Y., Matsuoka, M., Torii, K, Hayashi, H., Takii, T., Oomoto, Y. and Ona- zaki, K. Differential production of interleukin 1 (IL-1), IL-6, tumor necrosis factor, and IL-1 receptor antagonist by human monocytes stimulated with Mycobacterium leprae and M. bovis BCG. Int. J. Lepr. 61(1993)609-618.
19. World Health Organization. Progress towards leprosy elimination. Wkly. Epidemiol. Rev. 72(1997)165-172.
1. Ph.D.; Centro de Investigacion en Inmunologia y Dermatologia, C.U.C.S., Universidad de Guadalajara/Instituto Dermatologico de Jalisco, Av. Federalismo Norte 3102, Guadalajara, Jalisco, Mexico 44220.
2. M.Cs.; Centro de Investigacion en Inmunologia y Dermatologia, C.U.C.S., Universidad de Guadalajara/Instituto Dermatologico de Jalisco, Av. Federalismo Norte 3102, Guadalajara, Jalisco, Mexico 44220.
3. B.A.; R. Centro de Investigacion en Inmunologia y Dermatologia, C.U.C.S., Universidad de Guadalajara/Instituto Dermatologico de Jalisco, Av. Federalismo Norte 3102, Guadalajara, Jalisco, Mexico 44220.
4. M.D., Centro de Investigacion en Inmunologia y Dermatologia, C.U.C.S., Universidad de Guadalajara/Instituto Dermatologico de Jalisco, Av. Federalismo Norte 3102, Guadalajara, Jalisco, Mexico 44220.
5. Ph.D., Instituto de Biologia Molecular en Medicina, G.U.C.S., Universidad de Guadalajara, Guadalajara, Jalisco, Mexico.
Reprint requests to Mary Fafutis-Morris, Ph.D. atthe above address or FAX: 52-3-672-2848; e-mail:ciinde@cencar.udg.mx
Received for publication on 13 August 1998.
Accepted for publication in revised form on 27 April 1999.