- Volume 67 , Number 3
- Page: 287–91
Nasal mucosa and skin of smear-positive leprosy patients after 24 months of fixed duration MDT: histopathological and microbiological study
ABSTRACT
The skin and nasal mucosa of 10 lepromatous leprosy patients who had completed 24 doses of fixed duration multidrug therapy (MDT) but who continued to be skin- smear positive for acidfast bacilli (AFB) were examined histopathologically. The nasal mucosa showed granuloma fractions that exceeded those seen in the skin specimens, signifying that activity in this region subsides much more gradually than the activity in the skin. Mouse foot pad studies done using T900r mice with an inoculum f rom the nasal mucosa biopsy specimens of these patients did not demonstrate any growth of Mycobacterium leprae, indicating that these bacilli were not viable. A skin specimen f rom one patient grew significant amounts of bacteria in the T900r mouse foot pad. These results show that 2 years of treatment with MDT would prevent dissemination of M. leprae f rom the nasal mucosa and, therefore, should preclude further transmission of the disease. It also indicates that viable bacteria might persist in the skin of patients, especially those with an initial bacterial index of >4+ who have completed 24 doses of regular MDT. Therefore, a more cautious approach to administering only 12 doses of MDT to highly positive multibacillary patients is suggested.RÉSUMÉ
Un examen histopathologique de la peau et la muqueuse nasale fut réalisé chez 10 patients souffrant de lèpre lépromateuse, après qu'ils ont pris les 24 doses de polyehimiothérapie (PCT) à durée fixe et qu'ils furent restés posititis à l'examen du suc dermique pour la présence de bactéries acido-alcoolo-ré sistantes (AAR). La muqueuse nasale a montré une proportion plus importane de granulomes par rapport à la peau, indiquant que cette localisation anatomique présentait und baisse d'activité de la maladie plus graduelle que la peau. Il n'y a pas eu de croissance de Mycobacterium leprae après inoculations de préparations de biopsies de muqueuses nasales dans des pattes de souris T900r, indiquant que ces bacilles n'étaient pas viables. Une quantité significative de bactéries poussèrent dans les pattes des souris T900r à partir d'un spécimen de peau d'un patient. Ces résultats montrent que 2 années de traitement utilisant la PCT permet de prévenir la dissémination de M. leprae à partir de la muqueuse nasale et de ce fait devrait prévenir la transmission de la lèpre. Ils indiquent aussi que des bactéries encore viables peuvent persister dans la peau de certains pateints, en particulier ceux ayant un indice bactérioscopique initial supérieur ou égal à 4, même s'ils ont pris 24 doses de PCT standard. De ce fait, il est suggéré d'avoir une attitude plus prudente que l'administration de seulement 12 doses de PCT chez les patients multibacillaires hautement positifs.RESUMEN
Se hizo el examen histológico de la piei y la mucosa nasal de 10 pacientes con lepra lepromatosa quienes, habiendo completado un programa de poliquimioterapia (PQT) de 24 dosis, continuaron mostrando bacilos ácido resistentes en extendidos de linfa cutânea. La mucosa nasal tuvo granulomas más extensos que los observados en la piei, indicando que la enfermedad en esta region cede más lentamente que la enfermedad en la piel. La inoculaeiôn en la almohadilla plantar de ratones T900r con material obtenido de biopsias de la mucosa nasal de estos pacientes no produjo infecciôn, lo que sugiriô que los bacilos 110 eran viables. Solo 1111 espécimen de piel de uno de los pacientes contuvo bacilos viables que infectaron la almohadilla plantar de los ratones T900r. Lstos resultados muestran que 2 anos de tratamiento con PQT son suficientes para prévenir la diseminaciôn de Mycobacterium leprae a partir de la mucosa nasal y para evitar la transmisiôn posterior de la enfermedad. También indican que en la piel do los pacientes con indices bacterianos iniciales de >4+ peuden persistir bacterias viables, aun cuando los pacientes hayan completado 24 dosis de tratamiento con PQT. Se sugiere que se considéré el riesgo de adminstrar solo 12 dosis de PQT a pacientes altamente baciliferos.The World Health Organization (WHO) in 1982 recommended that a standard regimen of multidrug therapy (WHO/MDT) composed of rifampin, dapsone and clofazimine be given for multibacillary (MB) leprosy patients for at least 2 years and be continued, wherever possible, up to smear negativity. This combined regimen was to be given until the size of the bacillary population had been reduced to such an extent that resistant mutants were no long present (9).
In 1993 the WHO Study Group on Chemotherapy of Leprosy suggested that the WHO/MDT treatment for MB leprosy be limited to 2 years, thus eliminating the provision that the regimen be continued, wherever possible, up to smear negativity (10). In 1997, the WHO Expert Committee on Leprosy felt that from an operational point of view the duration of MDT for MB leprosy was still very long, and was responsible for critically affecting the implementation of MDT among all patients needing treatment (11). After taking into consideration various factors it concluded that it was possible that the duration of the WHO/ MDT regimen for MB patients could be further shortened to 12 months. However, studies have revealed that a certain percentage of MB patients continue to exhibit smear positivity after completion of fixed duration MDT of 24 doses (2-5-7). Although the bactericidal capabilities of the drugs used in the WHO/MDT regimen have been described, a possibility exists that some organisms may survive the fixed duration MDT regimen, especially in those patients with an initial bacterial index (BI) of over 4+(3).
A few studies have investigated the viability of these organisms gathered from the skin and lymph nodes of smear-positive patients (6) but no study has looked at the possibility of viable bacteria remaining in the nasal mucosa of patients who have completed 24 doses of WHO/MDT. Such bacteria, if their existence can be proved, may constitute an important source for the dissemination of Mycobacterium leprae . This study was undertaken to determine whether viable bacteria are present in the nasal mucosa and skin of smear-positive MB patients who have completed the fixed duration WHO/MDT regimen of 24 regular doses.
MATERIALS AND METHODS
Ten consecutive lepromatous leprosy patients attending the outpatient clinic of the Schieffelin Leprosy Research and Training Centre, Karigiri, India, who had received WHO/MDT and who were skin-smear positive at the end of their antileprosy treatment were included in the study. Eight patients had received 24 doses of WHO/MDT and two patients had 35 and 50 doses of WHO/MDT, respectively. One of the patients had had 7 years of treatment with dapsone prior to being treated with WHO/MDT. Seven patients had repeated episodes of erythema nodosum leprosum (ENL) during the treatment period. Each of these patients, along with a complete clinical examination, had a skin-smear examination for acid-fast bacilli (AFB) from routine and selective sites done at the start and completion of WHO/MDT.
After completion of WHO/MDT (1 month after the last dose of rifampin was given), an elliptical piece of skin from one of the old lesions was biopsied and bisected. Simultaneously, a small fragment of mucosal tissue from both sides of the nasal inferior turbinate was removed. One half of the biopsied skin and the nasal mucosal tissue removed from the right side of the nose were sent for mouse foot pad (MFP) inoculation. The other half of the skin and nasal mucosal tissue from the left side of the nose were sent for histopathological examination.
Half of each of the skin biopsies were fixed in formol Zenker; the left side nasal biopsies in 10% neutral formalin. Sections, 5-µm thick, made from these biopsies were stained with hematoxylin and cosin (H&E) for routine histopathological examination and with a modified Fite-Faraco stain for detecting AFB.
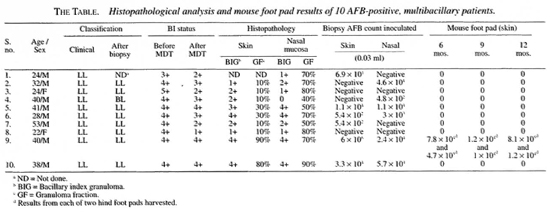
The other half of the skin biopsies and the nasal mucosal tissues taken from the right side of the nose were processed by the Rees method (8), and the bacteria isolated were injected into both foot pads of five immunocompromised T900r mice. The sizes of the inocula of M. leprae are given in The Table. Foot pad harvests and bactcrial counting were done at months 6, 9, and 12 after the date of inoculation.
RESULTS
Bacterial index (BI)
The pretreatment average bactcrial index (BI) was 4+ in eight of the patients and 3+ and 5+ in the other two patients. At the completion of antileprosy treatment, clinically, all of the patients showed signs of resolving lepromatous leprosy. The average BI of these patients immediately after the last dose of WHO/MDT ranged from 1+ to 4+ (The Table).
Histopathology
Skin. In most skin biopsies focal collections of foamy macrophages were seen in the dermis and, in some, the dermis was almost completely replaced by sheets of foam cells surrounding blood vessels and skin adnexal structures (Fig. 1). The granuloma fraction (GF) varied from 10% to 80% with an average GF of 31 %. The dermal nerves showed perineural thickening and fibrosis. Acidfast stained sections showed bacilli in all of the sections with a bacillary index granuloma (BIG) ranging between 1 + and 4+.
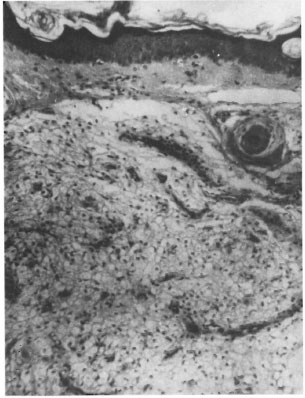
Fig. 1. Photomicrograph showing lepromatous granulomas composed of foamy macrophages surrounding a hair follicle. Epidermis shows atrophy (H&E x250).
Nasal mucosa. All of the nasal tissue sections showed varying degrees of atrophy, focal ulceration, and stromal collagenization. There was intense cellular infiltration with macrophages, polymorphs, lymphocytes and plasma cells (Figs. 2 and 3). The GF ranged from 40% to 80% with an average GF of 67%. The BIG varied from 1+ to 4+. In one of the nasal mucosa specimens no AFB could be detected.
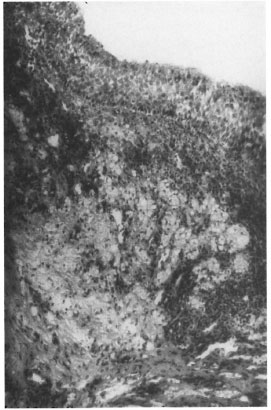
Fig. 2. Photomicrograph showing nasal mucosa lined by pseudo stratified columnar epithelium beneath which is seen a lepromatous granuloma (H&E x 100).
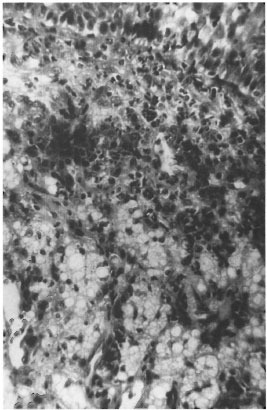
Fig. 3. Photomicrograph of a persisting lepromatous granuloma composed of foam cells, lymphocytes, polymorphs and plasma cells (H&E x 250).
Mouse foot pad studies
No bacterial growth was found in the foot pads of any of the T900r mice inoculated with suspensions prepared from the nasal mucosa of the 10 patients. There was no bacterial growth in any of the mouse foot pads inoculated with suspensions prepared from the skin biopsies of patients with one exception. One of the skin biopsy inoculated harvests showed an average bacterial growth of approximately 105 at months 6 and 9 and 106 at month 12 without corresponding growth in the nasal homogenates.
DISCUSSION
The two main portals of exit of M. leprae are the skin and the nasal mucosa. From all available evidence it is clear that of these two exit portals, the nasal mucosa is considered to be the more significant (4). M. leprae from nasal secretions have been found to remain viable outside the human host for up to 9 days (1). Histopathological study done on the nasal mucosa of the 10 lepromatous patients who continued to be skin-smear positive for M. leprae after completion of WHO/MDT showed that the granuloma fraction in the nasal mucosa was higher than that of the skin.
Clinically, even though the picture was one of resolving lepromatous leprosy at the end of 2 years of therapy, histopathological activity in the form of granulomas continued to exist in the nasal mucosa and, to a lesser extent, in the skin. This study demonstrates that despite the persistence of large amounts of AFB in the nasal mucosa of most MB patients who have completed their WHO/MDT, the persisting bacteria with the inoculum sizes used do not exhibit the capacity to grow and multiply in the foot pads of even immunocompromised mice. It is, therefore, reasonable to assume that WHO/MDT given for 2 years may be capable of sterilizing the viable M. leprae in the nasal mucosa and thereby would contribute greatly to the prevention of dissemination of live M. leprae . It also generally supports the validity of stopping WHO/MDT after 24 doses of regular treatment, irrespective of the presence of bacilli in the skin and nasal smears.
It is, however, disconcerting that 1 out of the 10 skin specimens grew AFB in the mouse foot pad. This specimen was taken from a patient whose pretreatment BI was 4+. This shows that viable M. leprae may persist in the skin of some of the smear-positive MB leprosy patients even after completion of 24 doses of regular WHO/MDT, especially if the BI is > 4+.
Given the widespread concern regarding the efficacy of the 12-month WHO/MDT MB regimen for the treatment of patients whose initial BI is >3+, the current study should be repeated in a similar patient group. This repeat study should be larger and should include patients with a BI of <3+ as a control group.
Acknowledgment. The authors thank Mrs. Shantha Arumugam for her technical help.
REFERENCES
1. Desikan, K. V. Viability of Mycobacterium leprae outside the human body. Lepr. Rev. 48(1977)231-235.
2. Ganapathi, R., Revankak, C. R. and Rashmi, R. P. Three years assessment of multidrug therapy in multibacillary leprosy cases. Indian J. Lepr. 57(1987)44-49.
3. Marchoux Chemotherapy Study Group. Relapses in multibacillary leprosy patients after stopping treatment with rifampin-containing combined regimens. Int. J. Lepr. 60(1992)525-535.
4. Noordefn, S. K. The epidemiology of leprosy. In: Leprosy. 2nd edn. Hastings, R. C., ed. Edinburgh: Churchill Livingstone, 1994, p. 37.
5. Pattyn, S. R., Bourland, J., Grillone, S. and Janssens, L. Multibacillary leprosy can be treated by a fixed duration therapy. (Abstract) Int. J. Lepr. 57 Suppl.(1989)428.
6. Sivaprasal), N., Snehalatha, S., Lobo, D., Aschhoff, M. and Job, C. K. Viability of Mycobacterium leprae in lepromatous patients after 5 years of dapsone monotherapy supplemented with 2 years of multidrug therapy. Indian J. Lepr. 67(1995)427-433.
7. THELEP Subcommittee on Clinical Trial oe Chemotherapy of Leprosy. Scientific Working Group UNDP/World Bank WHO Special Programme for Research and Training in Tropical Diseases. Persisting M. leprae among THELEP trial patients in Bamako and Chinglepet. Lepr. Rev. 58(1987)325-327.
8. WHO document. TDR/SWG- THELEP (1)/77.3, Annex I, Appendix 5.10. Geneva: World Health Organization, 1977.
9. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982, p. 23. Tech. Rep. Ser. 675.
10. WHO Study Group. Chemotherapy of leprosy. Geneva: World Health Organization, 1994, p. 12. Tech. Rep. Ser. 847.
11. WHO Action Programme for the Elimination of Leprosy. Status report updated. Geneva: World Health Organization, 1997, pp. 7-10. WHO/LEP/97.4.
1. M.B.B.S., M.D., Head, Department of Histopathology and Experimental Pathology, Schieffelin Leprosy Research and Training Center, Karigiri, Vellore District, Tamil Nadu, India 632 106.
2. M.B.B.S., M.S., D.L.O., Head, Department of ENT, Christian Medical College and Hospital, Vellore District, Tamil Nadu, India 632 004.
3. M.B.B.S., Medical Officer, Branch of Medicine; Schieffelin Leprosy Research and Training Center, Karigiri, Vellore District, Tamil Nadu, India 632 106.
4. M.B.B.S., M.D., Head, Branch of Medicine; Schieffelin Leprosy Research and Training Center, Karigiri, Vellore District, Tamil Nadu, India 632 106.
5. M.A., M.P.H., F.S.S., Dr.PH., Director; Schieffelin Leprosy Research and Training Center, Karigiri, Vellore District, Tamil Nadu, India 632 106.
6. M.B.B.S., M.D., F.R.C. Path., F.A.M.S., Emeritus Scientist, Schieffelin Leprosy Research and Training Center, Karigiri, Vellore District, Tamil Nadu, India 632 106.
Received for publication on 18 February 1999.
Accepted for publication in revised form on 27 April 1999.