- Volume 67(4 Suppl 1) , Number 4
- Page: 1–82
Workshop on the prevention of leprosy Pohnpei Federated States of micronesia 25-27 May 1999
Sponsored by the
Sasakawa Memorial Health Foundation
Tokyo, Japan
and the
Western Pacific Regional Office
of the
World Health Organization
Geneva, Switzerland
Editor for this Supplement is
Louis Levy, M.D., Ph.D.
Visiting Professor,
Department of Dermatology,
Hadassah University Hospital,
Jerusalem, Israel
INTRODUCTORY REMARKS
Yo Yuasa
Sasakawa Memorial Health Foundation Tokyo, Japan.
Good morning, ladies and gentlemen. I think we are ready to begin this Workshop on the Prevention of Leprosy. I think this is a very interesting and timely subject, as we approach the important milestone in our fight against leprosy-eliminating leprosy as a public health problem on a national scale. In this fight, Micronesia faced some difficulties, because of a very high prevalence and incidence of leprosy. The Western Pacific Regional Office (WPRO) of the World Health Organization and the Sasakawa Memorial Health Foundation (SMHF) cooperated in supporting a program of preventive chemotherapy in this country. Thanks to Dr. Eliuel Pretrick and his staff, this program was completed successfully, and, this morning, we shall hear the results of their efforts. Also, because of their success, similar programs have been instituted in this region-in the Marshall Islands and Kiribati, and we shall also hear about these programs.
There are many ways to continue our fight against leprosy, working toward a world without leprosy. Prevention of leprosy is one important aspect of our strategy, if it will be at all possible to reach this goal. We are happy to have with us Dr. M. D. Gupte, who is an expert in immunoprophylaxis; he has recently completed a very successful trial of immunoprophylaxis in India, and we look forward to his paper. But in this Workshop, chemoprophylaxis will be the major topic, and we are happy to have with us a number of experts in this area. We look forward to a very fruitful Workshop over the next two-and-one-half days, and hope to produce ideas and suggestions for our colleagues who are working to control leprosy in their countries.
This Workshop is sponsored by the SMHF, co-sponsored by WPRO, and hosted by the government of the Federated States of Micronesia (FSM), and we are honored to have with us this morning Dr. Pretrick, who has been our collaborator for nearly 20 years in the fight against leprosy in this country.
This is a very small Workshop in terms of the number of participants, but very rich in expertise in leprosy. The papers to be presented will be published as a Supplement to the International Journal of Leprosy, and, thus will be available to a much larger audience, so that this Workshop will be very important in guiding leprosy-control activities in the future. I look forward to very interesting and fruitful outcomes of the Workshop.
WELCOMING REMARKS
Eliuel K. Pretrick
Secretary of Health and Education Pohnpei, Federated States of Micronesia.
It is indeed a great honor and privilege for me, on behalf of the government and the people of the Federated States of Micronesia (FSM), to welcome to Pohnpei the participants in this important Workshop on the Prevention of Leprosy. As you know, the FSM is a small island nation, which gained its independence 13 years ago. Among a total of 670 islands scattered over an area of 1.8 million square miles of ocean, 60 islands are inhabited. The total landmass is a mere 271 square miles, with a population of 105,000.
At the outset, I wish to thank the organizers-the Sasakawa Memorial Health Foundation (SMHF) and the Western Pacific Regional Office of the World Health Organization (WHO) for choosing the FSM as the venue of this workshop, in which so many experts in the field of leprosy from different parts of the world are participating. That you have chosen the FSM as your meeting place is particularly fitting, because the FSM has been experiencing one of the highest prevalence rates of leprosy in the world.
Leprosy patients were present in the FSM well before independence. Here in Pohnpei State, leprosy was first noted on the island of Pingelap, an atoll of 0.26 square miles of land area and a population of 800, 160 miles east of the island of Pohnpei. A Pingelapese laborer who had returned from working in the phosphate mines of Nauru began to develop skin lesions on his body. He later died and was buried on the island. Some years later, similar skin patches began to be noticed on more people living on the island of Pingelap and in Sokehs municipality, a Pingelapese village here on Pohnpei. They were suspected as leprosy, and the patients were isolated on a small island on the reef adjacent to Mwolok village.
It is also our recollection that, on account of leprosy, diagnosed cases were sent to an island in the Marshall Islands for isolation and care. They were given a one-way ticket, because they were expected to remain there for the rest of their lives, there being no hope of cure for the disease. Later, during the American administration, they were allowed to return home.
Immediately after World War II, because of the increasing number of leprosy patients, the patients were isolated on Saputik, a larger island. Later, other patients from Chuuk, Yap, Palau, the Marshall Islands and Saipan joined the patients of Saputik Island in a leprosarium established on the island of Tinian in the Northern Marianas. Eighty percent of the patients were from Pohnpei, and 80 percent of the Pohnpei patients were from Pingelap. This leprosarium was not operated for very long; by 1962, most of the patients had been sent back to their home islands for continuing isolation and care.
By 1960, both leprosy and tuberculosis were important public health problems throughout these islands. A mass campaign for tuberculosis was carried out, in which all residents were tested with PPD, and BCG was administered to all PPD non-reactors. During the early 1980s, leprosy remained a public health problem, whereas the prevalence of tuberculosis decreased. Pohnpei State continued to experience a high prevalence of leprosy, with more new cases found among ethnic groups other than the Pingelapese- i.e., Pohnpeian and the people of Kapingamarangi, another atoll belonging to Pohnpei State, and in other states of the FSM. In summary, leprosy has been recognized as a public health problem in the FSM for many years. The reported prevalence is about 34 per 10,000 population, one of the highest in the world.
A work plan was developed in 1984 in collaboration with the U.S. Public Health Service (USPHS), the WHO, and the SMHF. The USPHS Hospital in Carville and the University of Hawaii School of Public Health were also involved. Multidrug therapy (MDT) as recommended by the WHO was introduced, with financial and other assistance by the SMHF.
Following the introduction of MDT, there was a steady decline of the number of new cases detected. However, the prevalence appeared to have leveled off during the four years prior to the institution of preventive treatment in the FSM two years ago. There appears to have been a high rate of default among those administered MDT; this could be one of the factors that caused the case-detection rate to remain relatively constant during the years of MDT prior to launching the Leprosy Prevention Project.
After a group of experts visited the FSM to evaluate our situation and advise us, the FSM requested that the Leprosy Elimination Project be carried out. The Project was launched 16 May 1996 in Mwolok village, with the goal of decreasing the prevalence to 1 per 10,000 population, and was completed in May 1998. We await evaluation of the report of the Project. Now, in May 1999, exactly one year after completion of the Leprosy Elimination Project, it is entirely appropriate that this important Workshop on the Prevention of Leprosy be held in the FSM; we hope to learn more about the impact of the Leprosy Elimination Project on the problem of leprosy in the FSM. In honor of this occasion, we have declared 25 May 1999 to be FSM Leprosy Day.
Again, on behalf of the government of the FSM, I welcome you and express our sincere gratitude for the assistance provided by the WHO, the SMHF and this group of experts. I hope you all enjoy your brief stay in Pohnpei during this Workshop.
WELCOME F ROM THE WORLD HEALTH ORGANIZATION
Leopold J. Blanc
Western Pacific Regional Office World Health Organization Manila, The Philippines.
In the Western Pacific Region of the World Health Organization (WHO), which has a population of 1.6 billion, there are approximately 20,000 cases of leprosy, yielding a prevalence of almost zero, and there are approximately 10,000 new cases annually. However, these are mean figures, which hide the real problems. In a few small countries, such as the Federated States of Micronesia (FSM), Republic of the Marshall Islands (RMI) and Kiribati, and to some extent Guam, the prevalence of leprosy is still very high, and the trend of new-case detection has changed very little, despite the introduction of multidrug therapy (MDT). This prompted the governments and the WHO to seek answers to the continuing high detection rates at the moment that leprosy is being eliminated from the world as a public health problem.
Screening of entire populations to identify cases of leprosy was proposed, as was administration to the apparently healthy members of those populations of a chemoprophylaxis that had proven effective in earlier trials. The operation began in the FSM as a component of the program of leprosy control, and not as an experimental trial. In the Workshop on Elimination of Leprosy, sponsored by Western Pacific Regional Office (WPRO) of the WHO and held in Manila in June 1998, the manager of the leprosy-control program described the plan and the work carried out in the FSM. This presentation stimulated proposals from neighboring countries to carry out similar activities.
I wish to congratulate the FSM for having completed this very large undertaking, which required screening the entire population twice in two years. Today, experts from around the world have gathered here to share their knowledge of chemo- and immunoprophylaxis. I wish also to thank the government of the FSM, which has hosted this Workshop. And I hope that the Workshop will produce recommendations for the most effective means of employing chemoprophylaxis and vaccination, in order to guide leprosy-endemic countries in the future use of these tools.
TRIALS OF PREVENTIVE THERAPY
Leopold J. Blanc
Western Pacific Regional Office World Health Organization Manila, The Philippines.
In five countries in the Western Pacific Region of the World Health Organization- the Federated States of Micronesia (FSM), the Republic of the Marshall Islands (RMI), Kiribati, Papua New Guinea and Guam, the prevalence of leprosy is still greater than 1 per 10,000 population. The continuing high prevalence of leprosy in these countries has kindled renewed interest in chemoprophylaxis.
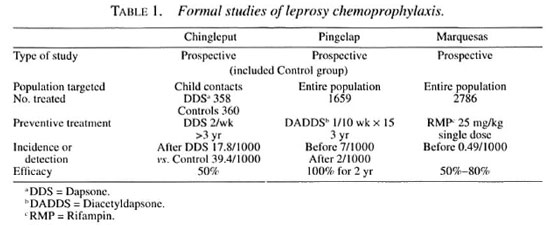
A number of formal studies of chemoprophylaxis have been carried out in the past, and I wish briefly to review three of them. Dr. S. K. Noordeen carried out a trial in Chingleput, India, in 1965, in which dapsone (DDS) was administered orally to child contacts of patients with leprosy. Subsequently, based on the results of this trial, a trial was carried out by the U.S. Public Health Service and the University of Hawaii on the island of Pingelap and in the Pingelapese community on the island of Pohnpei, as has been mentioned by Dr. Pre-trick, employing injectable diacetyldapsone (DADDS), which was administered to the entire population. The third trial about which I wish to speak was carried out by Dr. Jean-Louis Cartel in French Polynesia, in the South Marquesas Islands, employing a single dose of rifampin, which also was administered to the entire population.
As shown in Table 1, all three of the trials were prospective studies. Only in Chingleput was a control group included; the results of this trial were sufficiently convincing to render the later inclusion of a control group unethical. In the Chingleput trial, only the children who were contacts of patients with multibacillary (MB) leprosy were treated, whereas the trials in both Pingelap and the Marquesas targeted entire populations. In Chingleput, 700 contacts were treated, whereas larger numbers were treated in both the Pingelap and South Marquesas trials. In both the Chingleput and Pingelap trials, the prophylaxis was administered for at least three years, a very long time, whereas the prophylaxis was administered in only a single dose in the South Marquesas trial. The prevalence of leprosy before beginning the trial was very high among household child contacts in Chingleput, and also high in the total population in Pingelap, whereas it was considerably lower, although still well above the target of 1 per 10,000 population in the South Marquesas Islands. It must be remembered, of course, that the Chingleput and Pingelap trials were conducted well before the introduction of multidrug therapy (MDT), whereas that in French Polynesia was conducted against a background of a declining rate of detection of new cases after MDT had been introduced. In Chingleput, the efficacy of the prophylaxis was estimated to be 50 percent, comparing the incidence among treated and control subjects. In Pohnpei, no new cases appeared during the first two years after the course of prophylaxis, but new cases were observed later. During the four years following the trial in the South Marquesas Islands, the new-case detection rate was reduced by 80 percent, in comparison with that recorded before the trial; however, because the prophylaxis was administered against a background of declining incidence, the result of MDT, the efficacy of the treatment was calculated to be of the order of 50 percent.
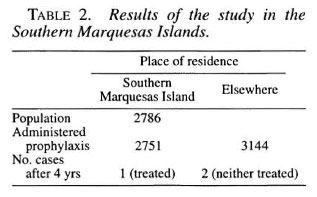
With respect to the trial in French Polynesia, the population of the South Marquesas Islands was 2786, 98.7 percent of whom received prophylaxis. As shown in Table 2, however, in addition to those residing on the Island, the members of the population who were living off the Island, numbering more than those residing on the Island, were also treated in an attempt to "sterilize" the reservoir of Mycobacterium leprae. During the four years following administration of the prophylaxis, one patient with a single lesion, who had been administered the prophylaxis, was encountered among those living on the Island, obviously a case of failure of the treatment. Among those residing off the Island, two patients with MB leprosy were detected, neither of whom had been administered chemoprophylaxis.
In addition to these formal trials, chemoprophylaxis has recently been introduced in three countries-the FSM, Kiribati and the RMI-as a component of their leprosy-control programs, in reaction to the failure of new-case detection rates to fall (The Figure). In these three countries, entire populations rather than the population of a single island have been screened, and chemoprophylaxis has been administered either to the entire population or to household contacts. The second round of screening has not been completed in Kiribati, and even the first round has not yet been completed in the RMI, whereas the program in the FSM was completed only one year ago so that only preliminary data are available for presentation at this time. These programs will be described in detail in the following papers.
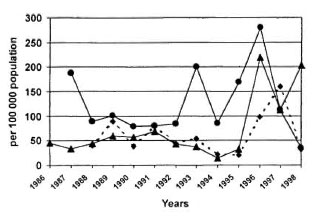
The figure. New-case detection rate in three Westcrn Pacific countrics, 1986-1998. • = Kiribati;• = FSM; • = RMI.
DISCUSSION
Dr. Noordeen: Can you tell us whether case detection rates in the Western Pacific Region have changed over the last number of years?
Dr. Blanc: In the large countries of this Region, case-detection rates didn't change much over the last 10 years, until last year. In 1998, for the first time, we experienced a dramatic decrease from approximately 13,000 per year to 10,000, despite the Leprosy Elimination Campaigns (LECs), Special Action Projects, and mass screening. Thus, I do not believe that the decrease of case-detection rates is an artifact, resulting from decreased case-finding activities. Two countries have been the main contributors to this decrease. In The Philippines, LECs were carried out in six provinces, and hitherto unreached areas have been reached. In Cambodia, three LECs were organized in 1998. Not only did the number of new cases decrease, but the proportions of patients with disability and of MB patients also decreased. In fact, although the number of new cases declined for the first time in 1998, the decreases of the proportions of patients with disability and of MB patients represent trends.
ELIMINATION OF LEPROSY IN THE FEDERATED STATES OF MICRONESIA BY INTENSIVE CASE FINDING, TREATMENT WITH WHO/MDT AND ADMINISTRATION OF CHEMOPROPHYLAXIS
Carmine Diletto
Western Pacific Regional Office World Health Organization Manila, The Philippines.
The Federated States of Micronesia (FSM) comprises 607 islands, of which 65 are inhabited. The islands are spread across more than a million square miles of the western Pacific Ocean. Four states are included in the Federation-Pohnpei, Chuuk, Yap and Kosrae. Vast distances and a lack of transportation between islands are important characteristics of the country.
Current population estimates are based on a census carried out in 1994, in which 105,506 people, including emigrants, were enumerated. Between 1986 and the present day, more than 11,000 Micronesians have migrated to Guam, Hawaii, the Commonwealth of the Northern Mariana Islands (CNMI) and the continental U.S. The 1994 census data have been used to calculate the completeness of the coverage by screening and chemoprophylaxis.
During the past 10 years, although the prevalence rate of leprosy has been declining steadily, the new-case detection rate has remained stable at 10 per 10,000, with peaks of 21 in 1993 and 19 per 10,000 in 1995, years in which active case finding was carried out (The Figure). A survey of selected villages in Pohnpei and Chuuk, conducted in 1995, resulted in the detection of 71 new cases among a population of 10,865, indicating that the new-case detection rate might be as high as 65 per 10,000. Moreover, the same survey demonstrated that 62 per cent of the new cases were among children under 15 years of age, suggesting a very high rate of transmission of Mycobacterium leprae in this community. Given this alarming situation, and in keeping with the commitment of the country and of the World Health Organization (WHO) to eliminate leprosy by the year 2000, a technical discussion was held 27 November 1995 in Manila, The Philippines, to discuss the situation in the FSM, and to plan appropriate action. The resulting plan included two rounds of screening of the entire population to detect and treat all existing cases by the multidrug therapy recommended by the WHO (WHO/MDT). It was also planned to administer chemoprophylaxis to all healthy individuals, with the hope that this would prevent the development of the disease among those already infected. Chemoprophylaxis would be administered twice, one dose for each round of screening. The combination rifampin-ofloxacin-minocycline (ROM) was to be administered to adults in the doses: 600 mg rifampin, 400 mg ofloxacin and 100 mg minocycline. Children under 15 years of age would be administered rifampin alone in the dosage of 25 mg per kg. To monitor the efficacy of the chemoprophylaxis in the near term, the use of immunological tests was proposed. A rapid survey, to be conducted six months after completion of the project, was also suggested.
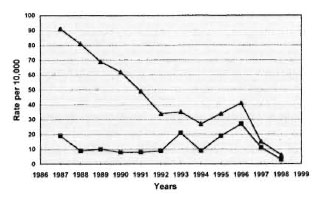
THE FIGURE. Leprosy prevalence and new-case de-tection rates in the FSM, 1986-1998. • = Prevalenceper 10,000; • = new-case detection rate per 10,000.
This plan became the basis for the development of a joint project among the government of the FSM, the WHO and the Sasakawa Memorial Health Foundation (SMHF). To assist in implementation of the project, the WHO assigned two consultants, one based in Pohnpei State and the other in Chuuk State. The author, based in Pohnpei, was assigned to the project from the beginning of second round.
To carry out the project, a mobile leprosy team was formed in each of the four states, the members of which were first trained according to their functions. Team leaders, all of whom were physicians, supervised the teams. Each team worked full time in implementing the project, going from village to village and island to island to screen the entire population.
Mass campaigns employing radio messages and meetings with community and government leaders preceded the operations. A health educator visited the villages before the screening to prepare the population and, with the participation of the community leaders, to organize the activities. Forms were prepared on which were recorded the names of those screened and those administered chemoprophylaxis, together with other relevant information. Forms for monthly reporting and for the new cases detected were also prepared. The populace usually met in an established meeting place, where the screening took place. If attendance was small, or if there were numbers of people that could not reach the screening point, the teams conducted home visits. Chemoprophylaxis was administered at the time of screening, under the direct supervision of a team member, whenever possible. It was necessary, on some occasions, especially during visits to homes in remote places, to leave the medications with a family member for some people who were absent; thus, it is possible that some who were recorded as having been administered chemoprophylaxis had not in fact been treated. Schools, private and government offices, and commercial and industrial establishments were also visited to screen those present. The most common skin diseases were also treated, and a portable emergency medical kit was also available to treat the side effects of chemoprophylaxis. When a patient with suspected leprosy was encountered, diagnosis and classification were performed by the team leaders, leprosy coordinators and WHO consultants; all cases that were checked by the author were confirmed as leprosy.
The first round began in March 1996 and ended in March 1997, except in Chuuk State; here, the first round was completed in June 1997. The second round began in March 1997 and was completed by the first half of May 1998. Because these were smaller states, only three to four months were required for each round in Yap and Kosrae.
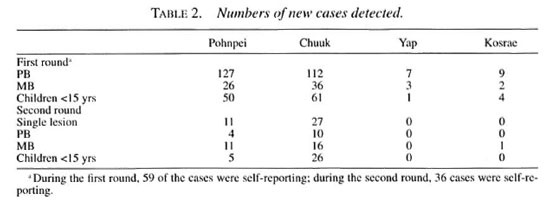
During the first round, 72 per cent of the population of the FSM was screened, and chemoprophylaxis was administered to 97 percent of those screened (Table 1). Although the number screened fell short of the target of 80 percent, those screened represent 78 per cent of those available for screening, if the 11,000 emigrants are subtracted. A total of 322 new cases were detected, of which 67 (21 percent) were multi-bacillary (MB) and 116 (36 percent) were under 15 years of age (Table 2). None of the new cases demonstrated disability of WHO grade 2.
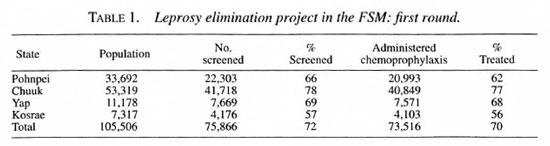
During the second round, 73 percent of the population was screened, and chemoprophylaxis was administered to 98 percentof those screened, as shown in Table 3. Ex-cluding the emigrants, 78 percent of the res-ident population was screened. During thisround, 18,731 people who had not beenscreened in the first round received chemo-prophylaxis for the first time. Eighty newcases were detected during the secondround, of which 35 percent were MB, 47 percent presented just a single lesion, and 39 percent were children under 15 years of age (Table 2). As in the first round, none of thenew cases demonstrated grade 2 disability.
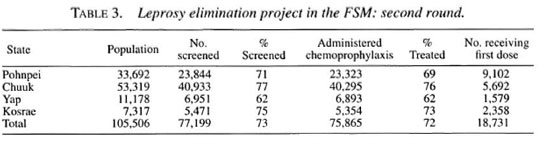
Combining the two rounds, 90 percent ofthe people were screened at least once, and55 per cent were screened twice; 87 percentwere administered at least one dose ofchemoprophylaxis, and 54 percent receivedtwo doses. Accounting for the emigrants,94 percent of those resident in the FSM re-ceived one dose of the chemoprophylaxis,and 58 percent received two doses.
That 322 new patients were detected inthe first round and only 80 in the secondround represents a 75 percent reduction ofthe number of new cases from the first tothe second round. Of the 80 new cases de-tected in the second round, 12 were re-ported to have received chemoprophylaxis during the first round, and must be consid-ered treatment failures. However, it must benoted that, for cultural reasons and becauseof diffìculties in finding private places forscreening, as well as inadequate light insome situations, the clinical examinationwas not always accurate, causing somecases to be missed. Missed cases were moreevident in the first round, because they weresubsequently detected in the second round.In fact, in Pohnpei, eight of the new casesthat were detected in the second round hadnoted their lesions well before the firstround of screening. This was also true foreight new cases in Chuuk and three in Kos-rae. And other patients detected in the sec-ond round may have been missed in the first.
Additional evidence for an effect of theintervention is that, in 1997, 122 new caseswere detected, representing a detection rateof 11 per 10,000 (The Figure), and prelimi-nary data for 1998 indicate a new-case de-tection rate of only 3 per 10,000, a reduc-tion of 89 percent from the rate of 1996.
Between 1996 and 1997, 488 patientscompleted treatment, as the result of whichthe prevalence decreased from 41 per 10,000 population in 1996 to 15 per 10,000 in 1997. Preliminary data for 1998 indicate a further reduction of the prevalence to 6 per 10,000. The sharp decline of the prevalence resulted also from the implementation, in late 1997, of the new simplified WHO/MDT regimens, which include a single dose of rifampin plus ofloxacin plus minocycline (ROM) for single-lesion leprosy and shortening the duration of treatment to 12 months for the treatment of MB patients.
According to preliminary data, at the end of 1998, the prevalence of leprosy in the four states of the FSM was as follows. In Yap, all patients had completed MDT, and Yap has been the first state of the FSM to achieve the goal of elimination. In Kosrae, three patients were still receiving MDT, yielding a prevalence of 4 per 10,000. In Pohnpei: with 27 patients remaining under treatment, the prevalence was 8 per 10,000; in Chuuk, there were still 35 cases under treatment, with a prevalence of 6 per 10,000.
In summary, beginning in March 1996 the government of the FSM, in collaboration with the WHO and the SMHF, implemented a special project to screen the entire population of the country for leprosy twice, and to administer chemoprophylaxis at the time of screening to all healthy individuals, with the aim of reducing the prevalence of leprosy to less than 1 per 10,000 by the year 2000. The chemoprophylaxis consisted of ROM for adults and rifampin alone for children under 15 years of age. The project was completed by May 1998.
Eighty-seven percent of the healthy individuals received one dose of ROM, and 54 percent received two doses. Three-hundredtwenty-two new cases were detected during the first round (new-case detection rate 31 per 10,000) and 80 were detected in the course of the second round (detection rate 8 per 10,000)-a 75 percent reduction of the number of new cases from the first to the second round. Combining the two rounds, 402 new patients were detected of whom 24 percent were MB and 37 per cent children under 15 years of age. No patients with grade 2 disability were detected. Of the 80 new cases detected in the second round, 12-two from Pohnpei and 10 from Chuuk-were reported to have received chemoprophylaxis during the first round.
DISCUSSION
Dr. Noordeen: Do you have any difficulty linking new cases with your old records?
Dr. Diletto: There are some difficulties. For example, the data have not been computerized. The records have been filed according to the place in which the individual was screened. The new patient may state that he had received chemoprophylaxis, but this is difficult to verify without knowing where the screening took place.
Dr. Noordeen: Is any method employed in the FSM to identify individuals, such as identity cards?
Dr. Pretrick: No.
Prof. Ji: Your data suggest that a single dose of ROM is capable of reducing the risk of leprosy by approximately 90 per cent, comparing the new-case detection rate among those administered ROM with that among those not so treated.
Dr. Diletto: Dr. Blanc will have more to say on this point. However, I wish to point out that, in Kiribati, where no chemoprophylaxis was administered, the new-case detection rate decreased by 85 per cent from the first to the second round.
Prof. Ji: What is your next step?
Dr. Diletto: We were hoping that the experts gathered here would advise us on next steps. The original plan called for serological studies, about which Prof. Cho will speak, and a rapid survey to be carried out six months after the end of the second round; we certainly don't intend once again to screen the entire population. In fact, we have prepared a proposal, complete with budget, for a resurvey of several high-prevalence villages in Pohnpei State.
IMPLEMENTATION OF CHEMOPROPHYLAXIS IN POHNPEI STATE, FEDERATED STATES OF MICRONESIA
Elizabeth lehsi-Keller
Department of Health Services Pohnpei State Government, FSM.
The leprosy team, consisting of six members, was selected from among the health workers of the Department of Public Health. The team leader was a physician who had earlier been trained in clinical leprosy. All of the team members worked full time on the project. Before beginning operations, a week's training was given to the team, focusing on the methods of screening, diagnosis and treatment; the rationale of chemoprophylaxis and its administration; possible side effects and their management; public information and community participation; and recording and reporting.
Each of the team members had been assigned specific responsibilities, such as registration, physical examination for signs and symptoms of leprosy, administration of chemoprophylaxis and health education. Their activities were directed by the team leader and a consultant assigned by the World Health Organization (WHO). Physical examination and diagnosis were performed by the team leader or the WHO consultant. Diagnosis and classification were based on clinical findings, according to the criteria published in the WHO "Guide to Eliminating Leprosy as a Public Health Problem." Skin-smear examination for leprosy bacilli was available when necessary.
The schedule of field visits was prepared by the leprosy team, the WHO consultant and the leaders of the municipalities before beginning operations, and was updated monthly. The time required for screening the populace of each municipality was decided on the basis of the 1994 census and the geographical setting of the villages. The first round was begun in March 1996 and completed in February 1997. The second round was begun in March 1997 and completed in March 1998.
Before each visit of the leprosy team to a village, usually a few days before, meetings were held with the village leaders, and the populace was informed by means of radio messages and community meetings. On these occasions, information on the transmission of Mycobacterium leprae and the signs and symptoms of leprosy and its treatment, as well as information on chemoprophylaxis and its contraindications, were given to the populace. A WHO poster on diagnosis and treatment was displayed. Community centers, schools and health centers were used as sites to which the residents of the locality were invited by the village leaders for physical examination and administration of chemoprophylaxis.
Upon entry into the site, each resident was queried with regard to name, age, sex, the possibility of pregnancy and other contraindications such as renal and liver disease. The information was recorded on prepared forms, as was the outcome of the physical examination. The residents were examined individually by the team leader or the WHO consultant. When leprosy was detected, this information was recorded and, for new cases, a patient clinical card was opened and the treatment begun immediately. Information on the intake of the multidrug therapy (MDT), its duration and possible side effects was given to the patient.
After the screening, those among the populace who were eligible were administered chemoprophylaxis, the intake supervised by the responsible team member. Pregnant women were told to present to the Department of Public Health to take the medication after delivery. Those with present or past history of renal or liver disease were also excluded from chemoprophylaxis. Ointments for treatment of the most common skin diseases were also dispensed.
Home visits were conducted in each village, for those unable to attend, and when attendance at the screening site was small. During home visits, the team would split into three groups; one group stayed at the screening place and the other two conducted home visits in different parts of the village. Home visits were conducted more systematically and more frequently during the second round than during the first.
In the first round, according to the 1994 population census, 66 percent of the total population were screened, and 62 percent were administered chemoprophylaxis. In the second round, 71 percent of the population was screened, and 69 percent received chemoprophylaxis. Altogether, 89 percent of the population received one dose and 42 percent received two doses of chemoprophylaxis.
During the first round, 153 new patients were detected, of whom 26 (17 percent) were multibacillary (MB) and 50 (33 percent) were children under 15 years of age. During the second round, 26 new patients were detected, of whom 11 (42 percent) were MB, 11 (42 percent) demonstrated a single lesion, and 5(19 percent) were children under 15 years. The new-case detection rate in the first round was 45 per 10,000 and, in the second round, 8 per 10,000. Thus, the new-case detection rate decreased by 83 percent from the first to the second round.
The disease is unevenly distributed in Pohnpei State. During the first round, the prevalence was 45 per 10,000 population in Sokhes municipality, 275 per 10,000 on the outer island of Kapingamirangi and 193 per 10,000 on the outer island of Pingelap. Within Sokhes municipality, the villages of Kepira (15 new cases, 326 new cases per 10,000) and Kepin (4 new cases, 301 per 10,000) were the most affected. Within the municipality of Madolenihmw, the village of Metipw (9 new cases, 347 per 10,000) was the most affected.
DISCUSSION
Prof. Lechat: What is the total population of these high detection rate communities?
Dr. Keller: The population of Kepira is 460, that of Kepin 133, and that of Metipw 259.
IMPLEMENTATION OF CHEMOPROPHYLAXIS IN CHUUK STATE, FEDERATED STATES OF MICRONESIA
Junya Takashima
International Medical Center of Japan Tokyo, Japan.
Chuuk State is composed of more than 40 islands scattered over a wide area of the Pacific Ocean. Inside the lagoon are 15 populated islands, including the main island of Weno. In addition, there are five sets of outer islands. To the south are the Upper and Lower Mortlock Islands; the Western Islands lie to the west; and to the north and northeast lie the Hall Islands and Nomwin Atoll. Distances are vast; more than 100 miles separate the northernmost from the southernmost of the Western Islands, which are more than 150 miles distant from Weno, as are the Lower Mortlocks. Transportation among the lagoon islands is not difficult, but transportation among the outer islands is very limited and irregular.
The population of Chuuk State, the most populous of the four states of the FSM, was determined to be 53,300 in the 1994 census. The distribution of the population by island group and by age is shown in Table 1. Almost 50 per cent of the population is under 15 years of age.

The leprosy team, composed of eight members, was selected from among the health workers of the Department of Public Health. The team leader was one of the most active physicians in the health services. The team was given two days' training, which was focused on the methods of screening, diagnosis and treatment; the rationale of chemoprophylaxis and its administration; possible side effects and their management; public information; and recording and reporting.
For the operation, each of the team members was assigned a specific role-registration, examination for signs and symptoms of leprosy, administration of chemoprophylaxis, or health education. The activities were organized by the Director of Health Services and supervised by a consultant assigned by the World Health Organization (WHO). Physical examination and diagnosis were performed by the team leader or the WHO consultant.
Before beginning the operation, the schedule for field visits was prepared by the leprosy coordinator and the leprosy team, and was approved by the Director of Health Services. The time required for screening the populace of each island was determined on the basis of the 1994 population census and the distance from Weno.
Prior to the visit of the leprosy team, the population was informed through radio messages and a meeting with village leaders. In the case of the lagoon and outer islands, transmission of the information by the health workers from each island was also useful. On the day of screening, the leprosy team broadcast the information themselves, using a loudspeaker. Before beginning the screening, information on the transmission, signs, symptoms and treatment of leprosy as well as information on chemoprophylaxis and its contraindications was presented.
Community centers, schools and health centers were used as the places to which the populace came to be screened and to receive chemoprophylaxis. Village leaders or members of the community called the populace to meet at the screening point. A special place was prepared for skin examination, and bed sheets were hung as curtains to insure privacy.
Before the physical examination, the registrar queried those appearing to be screened for their name, age, and sex, and discussed with each individual the possibility of pregnancy and other contraindications, such as renal and liver disease. This information was recorded, as was the outcome of the physical examination.
Those presenting to be screened were examined individually by the team leader or the WHO consultant. When leprosy was detected, this information was relayed to the registrar, who recorded it on a specific form. In addition, for new cases, a patient clinical card was opened and the treatment begun immediately. The patients were informed with respect to intake of the multidrug therapy (MDT), its duration and possible side effects. For suspected cases, skin smear was performed if the suspect lived near the laboratory; if his home was far from the laboratory, he was instructed to present to the Department of Public Health three months later for reassessment. No patients were administered chemoprophylaxis.
After screening, those eligible were administered chemoprophylaxis under the supervision of the responsible team member. Pregnant women were instructed to present to the Department of Public Health after delivery to take the medications. Those with present or past history of renal or liver disease were also excluded from chemoprophylaxis.
Home visits were conducted in each village, usually for those unable to attend the screening and when screening coverage was low. During the home visits, the team would split into three groups: one would remain at the screening site, and the other two would conduct home visits in different areas of the village.
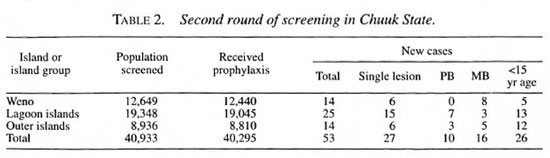
As is shown in Table 1, 40,755 people- 76 percent of the population-were screened in the course of the first round, and 39,751-98 percent of those screened -were administered chemoprophylaxis. During the second round, as shown in Table 2, 77 percent of the population was screened, and, again, 98 per cent of those screened were administered the drugs.
The results of the screenings, in terms of new cases of leprosy detected, are presented in Tables 1 and 2. In the course of the first round, 148 new patients were detected, yielding a detection rate of 27.8 per 10,000 at risk (Table 1), calculating the new-case detection rate on the basis of only those members of the population who were screened yields a rate of 36.3 per 10,000. A smaller number of new cases-53-was detected in the course of the second round of screening (Table 2), yielding new-case detection rates of 9.94 per 10,000, based on the 1994 census data, and 12.9 per 10,000, based on the number actually screened. Thus, new-case detection rates decreased by almost two-thirds, comparing the results of the two rounds of screening.
Side effects caused by the drugs used for chemoprophylaxis were rare. Only 29 individuals suffered mild cases of dizziness, skin eruption, nausea and chills. At the be ginning of the project, rumors of severe side effects were heard, but these soon disappeared and did not appear to influence the participation of the populace.
It was evident that the 1994 population census did not reflect the real situation in each village and municipality. Emigration to Guam, Hawaii, the Marianas and the U.S. was said to be very frequent. As a result, estimates of population coverage, prevalence and detection rate may be inaccurate, and a proportion of the resident population considerably larger than 76 percent may actually have been screened.
One patient in whom leprosy had been diagnosed earlier was found not to have leprosy, and his MDT was stopped. Eight patients had not been diagnosed during the first round, but were detected during the second round and placed on MDT.
DISCUSSION
Dr. Noordeen: Because you screened school children both in their schools and in their villages, is it possible that a child was given two doses of chemoprophylaxis, one when he was screened in school and the second if he were screened a second time in his village?
Dr. Takashima: Yes. This could have happened. However, most school children were screened in their villages, so that it usually was not necessary to screen school populations.
Dr. Izumi: How did you diagnose leprosy among children under five years of age? In my experience in Indonesia, this was often difficult.
Dr. Kyaw Tin: We saw a few patients with leprosy under five years of age in the RMI. The diagnosis was difficult; we tried to catch the child unaware, testing pin-prick sensitivity on the lesion and on normal-appearing skin.
MONITORING THE EFFECTS OF PREVENTIVE THERAPY IN THE FEDERATED STATES OF MICRONESIA
Sang-Nae Cho1; Gerald P. Walsh2; Patrick J. Brennan3
1. Department of Microbiology, Yonsci University College of Medicine, Seoul, Korea.
2. Leonard Wood Memorial Center, Cebu, The Philippines.
3. Department of Microbiology, Colorado State University, Fort Collins, Colorado, U.S.A.
The objective of this study was to assess the effects of the chemoprophylaxis of leprosy on Mycobacterium leprae transmission among residents of the FSM, by measuring the prevalence of antibodies to antigens of M. leprae among residents in FSM after the administration of chemoprophylaxis, and to attempt, by polymerase chain reaction (PCR), to detect M. leprae -specific DNA in nasal-swab samples obtained from among residents after chemoprophylaxis.
MATERIALS AND METHODS
A total of 3304 serum samples were obtained during the study period, including 1725 samples before chemoprophylaxis and 1125 one year after the first dose of chemoprophylaxis. Serum samples were obtained from two sources: from residents who presented to the leprosy team for screening and chemoprophylaxis; and from individuals who visited the health centers in Pohnpei, Chuuk and Kosrae States for reasons other than leprosy. Serum samples were stored frozen until examination for the presence of antibodies to M. leprae antigens.
To determine the proportions of individuals on whose nasal mucosa M. leprae -specific DNA could be detected by PCR, nasal-swab samples were obtained from those who presented to the leprosy team at the time of screening. A total of 1240 nasal-swab samples were obtained, including 629 samples obtained before chemoprophylaxis and 611 samples one year after the first dose of chemoprophylaxis. The surface of the nasal mucous membrane was wiped with a cotton-tipped swab which was then placed in L4 buffer and stored at room temperature until it could be transported to the laboratory.
RESULTS
First, sera from 1065 individuals who presented to the hospitals for reasons other than leprosy were examined. IgM antibodies to PGL-I, an M. leprae -specific antigen, were detected by ELISA using a neoglycoconjugate antigen, ND-O-BSA, which had been prepared in Fort Collins, Colorado, U.S.A. Using absorbance of 0.20 as the lower limit of seropositivity, the rate of seropositivity was 12.3 percent among residents of Pohnpei State, 13.9 percent in Chuuk State, and 5.9 percent in Kosrae State. The lower seropositivity rate in Kosrae State is consistent with the lower prevalence of leprosy in that state. The difference of seropositivity between Chuuk and Pohnpei States is not significant. The rate of seropositivity was highest among those aged 21-30 years (19.1 percent), followed by those aged 11-20 (15.1 percent) and the group aged 31-40 years (13.3 percent). That more than 13 percent of young children in Chuuk State were seropositive suggests that, before chemoprophylaxis, active transmission of M. leprae was still occurring.
Next, sera from 245 people, who presented to the health centers for reasons other than leprosy, and from 610 people one year after chemoprophylaxis were examined. The rate of seropositivity among those who had been administered chemoprophylaxis was 20.6 percent, virtually the same as the rate (20.0 percent) among a second group of 660 residents prior to chemoprophylaxis. The prevalence of anti-PGL-1 antibodies among those visiting the health centers was 17.5 percent, a bit higher than that during the first year in Pohnpei State. The rate of seropositivity was again highest among those aged 11-20 years, followed by that of the age group 21-30. These results suggest that young adults in Pohnpei State have the greatest chance of exposure to M. leprae, even one year after chemoprophylaxis. Another explanation is that anti-PGL-I antibodies decay only slowly
In an effort to investigate more directly the effect of chemoprophylaxis on the levels of anti-PGL-I antibodies, the sera of those who had taken the first dose of chemoprophylaxis and whose sera were obtained one year later were examined for changes of seroreactivity. A total of 81 paired serum samples were tested side by side. Of the 81 pairs, 21 (25.9 percent) were seropositive before chemoprophylaxis and 21 (25.9 percent)-not all the same pairs- were also seropositive one year after chemoprophylaxis, suggesting that there had been no significant impact on the rate of seropositivity by the first dose. However, some of the 81 demonstrated a substantial decrease of antibody level after chemoprophylaxis. Five individuals became seronegative after chemoprophylaxis, and the mean absorbance of the seropositive sera decreased from 0.41 to 0.37. Thus, chemoprophylaxis may result in a decline of the anti-PGL-I antibody level, although the difference of the means is not statistically significant. In addition, there were also some persons whose antibody levels increased after chemoprophylaxis, and five people became seropositive, suggesting that there may have been exposure to M. leprae after the chemoprophylaxis had been administered. Such a phenomenon is also consistent with continuing active transmission of M. leprae in the community.
In another effort to measure the effects of chemoprophylaxis, paired serum samples from high-school students in Pohnpei State and age-matched samples obtained from hospitals in Kosrae were examined in a randomized fashion for the presence of antibodies to MLSA and lipoarabinomannan (LAM), soluble antigens of M. leprae. There was no difference in the prevalence of antibodies to MLSA and LAM before and one year after chemoprophylaxis among high-school students in Pohnpei State: 24 percent were seropositive to LAM and 16 percent to MLSA. These results suggest that one dose of chemoprophylaxis had no significant effect on the prevalence of antibodies to M. leprae antigens.
In contrast, a marked decrease in the prevalence of anti-PGL-I IgM antibodies was found among the age-matched samples obtained from persons who presented to health centers in Kosrae State for reasons other than leprosy. The prevalence of IgG antibodies to MLSA or LAM changed little from before to one year after chemoprophylaxis. Despite the low rate of coverage (57 percent) of the program in Kosrae, the selective reduction of anti-PGL-I antibodies may reflect the efficacy of chemoprophylaxis among the residents of that state. An explanation for the difference in the prevalence of anti-PGL-I antibodies between Pohnpei and Kosrae States may be the difference of the prevalence of leprosy in the two states. The new-case detection rate in Pohnpei State was twice that in Kosrae State, suggesting that the residents of Pohnpei have a greater chance of re-exposure to M. leprae after chemoprophylaxis than do those of Kosrae. Further analysis using a larger sample size and samples obtained at longer intervals will be required to assess fully the effects of chemoprophylaxis on the kinetics of antibodies to M. leprae antigens.
Sera from 400 people who took chemoprophylaxis in the first or the second round were closely examined for changes in antibody levels to M. leprae antigens. Of 80 individuals who took two doses of the chemoprophylaxis and provided serum samples, there was no significant change in IgM antibodies to PGL-I. The mean and standard deviation of the absorbance of sera from the 80 were 0.26 ± 0.27 before chemoprophylaxis, 0.25 ± 0.25 one year after the first dose, and 0.25 ± 0.25 one year after the second dose, respectively. In some individuals, seropositivity decreased significantly, perhaps the result of chemoprophylaxis, but a larger number demonstrated no decrease and even an increase in seropositivity, despite two doses of chemoprophylaxis.
There were also 121 people who took only the first dose and from whom sera were obtained two years after the first dose. The mean and standard deviations of the absorbance of the sera from the 121 were 0.29 ± 0.32 before chemoprophylaxis and 0.27 ± 0.29 two years after the first dose; this small difference in seropositivity is not significant. In addition, there were 162 people who took only one dose and from whom serum samples were obtained one year after chemoprophylaxis. The mean and standard deviations of the absorbance of the sera from the 162 were 0.29 ± 0.31 before chemoprophylaxis and 0.26 ± 0.26 one year after the first dose. This small decrease in anti-PGL-I antibodies one year after chemoprophylaxis is not statistically significant.
In general, despite a marked decrease of the new-case detection rate in the FSM one year after chemoprophylaxis, there was no decrease in anti-PGL-I antibodies among those who had taken one or two doses of chemoprophylaxis. This stands in sharp contrast to the findings among leprosy patients under MDT. After MDT, anti-PGL-I antibodies decreased by 50 percent after two years of chemotherapy. Therefore, it may be that only one or two doses of chemotherapy (the chemoprophylaxis) are unable to kill all of the M. leprae in the body of the subclinically infected individual, or that the residents were exposed to M. leprae again after the chemoprophylaxis had been administered. Long-term monitoring of seroreactivity to the M. leprae-specific antigen, PGL-I, might indicate the degree to which M. leprae were being transmitted in the population.
A recombinant 45-kDa antigen, which contains M. leprae-specific epitopes, was also employed to assess the effect of chemoprophylaxis. The prevalence of IgG antibodies to the 45-kDa antigen among residents in Chuuk State was 5.4 percent before chemoprophylaxis, substantially lower than 13.7 percent, the prevalence of antibodies to the PGL-I antigen. When sera obtained from those who had taken two doses of chemoprophylaxis were examined for reactivity to the 45-kDa antigen, the mean seroreactivity in paired serum samples decreased from 0.45 before chemoprophylaxis to 0.34 one year after the first dose, and to 0.28 one year after the second dose. Thus, seroreactivity to the 45-kDa protein declined substantially after chemoprophylaxis. There are two possible explanations for the observation that the level of IgG antibodies to the 45-kDa antigen decreased more rapidly than did those of the antibodies to PGL-I and to LAM. It may be that antibodies to carbohydrate epitopes decay more slowly than do antibodies to protein antigens. Alternatively, antibodies to PGL-I or LAM are elicited more rapidly than are those to protein antigens. Whichever explanation is correct, this observation may be an indication that chemoprophylaxis will, in fact, reduce the transmission of M. leprae in the population, and that the new-case detection rate will decrease in the FSM in the near future.
A total of 1241 nasal-swab samples were examined for the presence of M. leprae DNA by a nested PCR. Of 629 samples obtained before chemoprophylaxis, 12 (1.9 percent) were PCR-positive, and 9 (1.4 percent) of 622 samples obtained one year after the first dose chemoprophylaxis were PCR-positive. This difference was not statistically significant.
CONCLUSIONS
The prevalence of anti-PGL-I antibodies among residents before chemoprophylaxis ranged from 5.9 percent in Kosrae to 13.7 percent in Chuuk State. In Chuuk State, the prevalence of anti-PGL-I antibodies was highest (19.1 percent) among those aged 11-20 years, followed by those aged 21-30 (15.1 percent), and least (13.3 percent) among residents under 10 years of age. There was little evidence of a decrease in prevalence of anti-PGL-I and anti-LAM antibodies two years after one or two doses of chemoprophylaxis. On the other hand, the prevalence of IgG antibodies to the 45-kDa antigen of M. leprae appeared to decrease one or two years after chemoprophylaxis. However, there was no significant decrease in the nasal carrier rate of M. leprae among residents determined by PCR one year after preventive therapy.
DISCUSSION
Prof. Ji: Can you compare the changes of seroreactivity among patients on MDT and healthy individuals administered chemoprophylaxis, as a measure of the efficacy of the chemoprophylaxis? Prof. Cho: I did not study any patient sera from the FSM. However, in other populations, we have found among patients on MDT that the degree of seropositivity decreases by about 30 percent after one year and by about 50 percent after two years. One must recognize that leprosy patients exhibit generally high degrees of seropositivity, with mean absorbance approximately 1.5; whereas those administered chemoprophylaxis in the FSM exhibited absorbance values under 0.5.
Prof. Levy: Prof. Cho has stated that his data indicate continuing infection. This is a very important point, because we are here to consider chemoprophylaxis. If we learned anything from the earlier studies of chemoprophylaxis, especially the trial in Pingelap, it is that chemoprophylaxis fails if the infectious patients are not treated and rendered noninfectious. Chemoprophylaxis may be expected to be effective against infections that have already occurred, but cannot be expected to be effective against future infections. Therefore, either these data do not support Prof. Cho's contention, or we have proof that, at least in the FSM, chemoprophylaxis was not effective.
Dr. Keller: How specific is PGL-I?
Prof. Cho: This appears to depend upon the population studied. In a nonendemic population, fewer than five percent may be seropositive.
POPULATION SCREENING AND MASS CHEMOPROPHYLAXIS IN KIRIBATI
E. C Daulako
P. J. Twomey Memorial Hospital, Suva, Fiji.
Kiribati is a country of 33 coral atolls and one volcanic island spread over a million square miles of the Pacific Ocean. Twenty-one of the islands are inhabited; the population projected for 1996 was 77,853. The Gilbert group consists of 16 inhabited islands, and the Line group of three; Phoenix Island and Banaba Island stand alone. Tarawa, in the Gilbert group, which is the capital island, contains about 40 percent of the population of the country.
Leprosy has been highly endemic in Kiribati (The Figure). When leprosy elimination activities were intensified in 1996, the number of new cases detected suddenly increased to 78, giving a new-case detection rate of 100 per 100,000 population. This situation prompted the government of Kiribati and its partners in leprosy elimination-the World Health Organization (WHO) and the Pacific Leprosy Foundation-to implement a project in which the entire population of the country was to be screened and chemoprophylaxis was to be administered to selected communities- those with very high endemicity and those which are geographically isolated. The project was implemented in order to accelerate progress toward the elimination of leprosy by the year 2000.
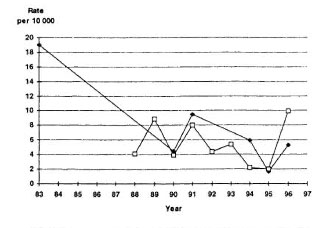
The figure. Leprosy prevalence and new-casedetection rates in Kiribati, 1983-1996. • = Preva-lence per 10,000: LI = new-case detection rate per10,000.
The project was implemented by the formation of three special teams. One team was led by the WHO Short-Term Consultant and each of the remaining two teams was led by a Leprosy Control Officer assigned by the Ministry of Health. The members of the teams were recruited from the health staff of the areas in which the teams were operating. The plan was to start with the Gilbert group of islands and subsequently to extend the activity to the Line group and Phoenix Island.
The project was preceded and accompanied by regular broadcasts on Radio Kiribati to inform the general public of what was being done and the expected outcome. Listeners were also told where the teams would be working during the week, to alert them to present themselves for examination.
The population was screened by house-to-house visits, and visits to schools and places of work; in addition, anyone encountered in the market place or along the roadside during the visit to a locality was examined. Diagnosis was made by the team leader at the time of screening, based on clinical examination. For newly detected patients, a record was opened and treatment was administered immediately.
For chemoprophylaxis, a combination of 600 mg rifampin, 400 mg ofloxacin, and 100 mg minocycline (ROM) was administered to adults (those above age 14 years), and rifampin alone was administered to children (those under 15 years of age); the dosage for children 10 to 14 years of age was 600 mg, for those 5 to 9 years 300 mg, for those 2 to 4 years of age 150 mg, and for children no older than 1 year 100 mg. Patients with leprosy, pregnant women, people with kidney or liver disease, and patients with tuberculosis whose treatment included rifampin were not administered chemoprophylaxis.
On Tarawa atoll, screening was begun 22 May and completed 31 July 1997. One to two weeks were required to complete the screening on each of the remaining islands. South Tarawa was screened for the second time between August and October 1998, one year after the first round of screening.
Chemoprophylaxis was administered to the entire population of South Tarawa and the Christmas Islands-the former, which contributed 73 percent of the active cases of leprosy in the country, because of high endemicity, and the latter because of its geographic isolation. Chemoprophylaxis was administered on South Tarawa at the time of the second round of screening; on Christmas Island, chemoprophylaxis was administered between 20 April and 6 May 1998, at the time of the first round of screening.
Screening of the population has been completed on 19 of the 21 inhabited islands-on all 19 islands of the Gilbert and Line groups of islands. The two remaining islands, Banaba and Phoenix, represent only 0.2 percent of the entire population of the country, according to the census of 7 November 1990; therefore, approximately 99.8 percent of the population of the country has been included. The population of the 19 islands covered by the project was estimated to be 76,624, of whom 70,638 (92.2 percent) were screened. The population of South Tarawa was screened for a second time; of the estimated population of 29,374, 26,536 (90.3 percent) were screened.
In the course of the first round of screening, 135 new cases were detected, yielding a new-case detection rate of 191 per 100,000 population. The highest rate was in South Tarawa, with 315 per 100,000, representing 69.6 percent of all detected cases, whereas five islands had no cases. The numbers of cases in the remaining islands ranged from one to seven. Of the new cases detected, 26 (19.3 percent) were multibacillary (MB), and 36 (26.7 percent) were under 15 years of age; there were no cases with disability.
During the second round, 15 new patients were detected, yielding a new-case detection rate of 51 per 100,000; among them were three MB patients, 11 patients with single lesions, and seven children under 15 years of age. Compared to the first round of screening, both the number of new patients and the detection rate were reduced by 84 percent.
In South Tarawa, 24,855 (84.6 percent) of the estimated population of 29,374 were administered chemoprophylaxis. Among those seen by the team, 1717 (6.9 percent) were not given the therapy for a variety of reasons. Adverse reactions were reported by 39 (0.16 percent) of those who received treatment. On Christmas Island, 2886 of the estimated population of 3271 (88.2 percent) were administered chemoprophylaxis; 209 (6.7 percent) were not given the therapy. Six (0.21 percent) of those administered chemoprophylaxis reported adverse reactions, all of them mild. For the two islands taken together, 27,741 (85.0 percent) of the estimated population of 32,645 were administered chemoprophylaxis, and 1926 (6.5 percent) were not given the therapy for a variety of reasons; 45 (0.16 percent) reported adverse reactions.
DISCUSSION
Dr. Diletto: How much time was involved in the first and second rounds of screening, and how much time elapsed between the two rounds?
Dr. Daulako: The first round required about 12 weeks, perhaps because we were inexperienced. Even though chemoprophylaxis was administered during the second round, we were familiar with procedures so it, too, required about 12 weeks. The time between the two rounds was one year.
Prof. Ji: Because your coverage during the first round of screening in South Tarawa was almost complete, it appears likely that most of the new patients detected in the course of the second round had been seen during the first. Do you think that most of the new patients seen in the second round had been missed in the first, or do you think that their disease developed during the interval between the two rounds?
Dr. Daulako: We certainly considered the possibility that the disease had been present during the first round. However, most of the new patients detected in the second round appeared to have early lesions, and to have developed their disease during the interval between the rounds.
Prof. Smith: Please clarify what was done in South Tarawa during the first and second rounds.
Dr. Daulako: During the first round, the population was examined, but no treatment was given. During the second round, the population was examined and chemoprophylaxis was administered.
Prof. Smith: Thus, the 85 percent reduction of the new-case detection rate from the first to the second round cannot be attributed to chemoprophylaxis. Perhaps, then, we should consider the detection rate during the second round as a more appropriate control, which may also be applicable to the FSM.
Dr. Daulako: Undoubtedly, many of the new patients detected during the first round had had symptoms and lesions for longer than one year, but had simply gone undetected.
Dr. Blanc: It is important to recognize that the reduction of the number of new cases detected resulted simply from cleaning up the backlog. In addition, it appears that the screening process was more efficient in Kiribati than in the FSM; thus, the new cases detected during the second round in Kiribati may represent only "incident" cases, whereas those detected during the second round in the FSM may represent a mixture of incident and backlog cases.
Prof. Smith: I'm fascinated by the lack of disability, despite a considerable proportion of MB cases. This is not ordinarily the case, and the explanation may be very important.
Dr. Noordeen: I concur. I was most impressed, during my earlier visit to the FSM, by the large proportion of MB patients, particularly among children, and the dearth of disability. In particular, I noted the virtual absence of trophic ulcers, which are an important problem among MB patients in other settings.
POPULATION SCREENING AND CHEMOPROPHYLAXIS FOR HOUSEHOLD CONTACTS OF LEPROSY PATIENTS IN THE REPUBLIC OF THE MARSHALL ISLANDS
Kyaw Tin
Consultant on Leprosy Control Yangon, Myanmar.
The Republic of the Marshall Islands (RMI) is a country of atolls and small islands spread over three-quarters of a million square miles in the central Pacific Ocean. Twenty-seven of the atolls and islands are inhabited, with a total population of 62,569 projected for 1998. About 68 per cent of the population live on Majuro and Kwajalein atolls; the remaining population is distributed among the outer islands, with populations ranging from around 100 to 2000. Majuro is the capital city of the country.
Leprosy is a serious public health problem in the RMI. The new-case detection rate for 1996 was 210 per 100,000 population, and the prevalence rate at the end of the year was 41 per 10,000; the corresponding rates for 1997, the year before this program was implemented, were, respectively, 115 per 100,000 and 27 per 10,000. Multidrug therapy (MDT) was available only on Majuro and Kwajalein atolls.
The government of the RMI aims to achieve elimination of leprosy, defined as reduction of the prevalence to less than 1 per 10,000 population, by the year 2000. The government planned to achieve this goal by detecting all cases in the country, and by making MDT available to the entire population. However, because of the high prevalence of the disease and the limited time remaining to achieve the goal, it was believed to be necessary to accelerate progress toward elimination. Therefore, it was planned to screen the entire population of the country to detect all current cases and to place them under treatment with MDT. At the same time it was planned to administer chemoprophylaxis to the household contacts of both past and current leprosy patients.
The program of population screening and chemoprophylaxis was implemented by the primary health care staff; program activities were incorporated into the routine activities of the staff and no additional staff was involved, except for a World Health Organization (WHO) Short-Term Consultant (STC) who visited the RMI periodically. The program was to be implemented simultaneously on Majuro and Kwajalein atolls. After completing the program on these two atolls, teams of staff members who had already gained experience were to be sent to the outer islands for implementation of the program there. The staff members who were to be involved in the program were given training in the tasks they were expected to perform. For diagnosis and classification of cases, the criteria presented in the "Guide to Eliminating Leprosy as a Public Health Problem," published by the WHO, were followed.
For chemoprophylaxis of household contacts, a combination of 600 mg rifampin, 400 mg ofloxacin, and 100 mg minocycline (ROM) was administered to those at least 15 years of age, and rifampin alone to children under 15 years of age. Children 10 to 14 years of age were administered 450 mg, those 5 to 9 years 300 mg, and those 1 to 5 years 150 mg. Pregnant women, children under one year of age, people with liver or kidney disease and those known to be allergic to any of these drugs were not given the drugs, nor were patients with past or current leprosy. Household contacts were defined as those living under the same roof and sharing household facilities with an index case (a patient with past or current leprosy).
Screening of the population of Majuro atoll was begun during the third week of May 1998. Zonal nurses made house-tohouse visits, listed the members of each household using a standard screening form, and examined available members for signs of leprosy. After all of the households had been visited, absentees were sought at their places of work or schools. Suspects were referred to the Public Health Division of the Ministry of Health, where the diagnosis of leprosy was confirmed or rejected by the Director of Public Health, Director of the TB/Lep Program, or by the WHO STC. The suspects who did not present themselves to the Public Health Division were sought again at their residences. The screening and follow up of suspects were completed in February 1999. Chemoprophylaxis for household contacts was delivered at their residences by the zonal nurses, beginning in January 1999 and finishing at the end of March 1999.
In Kwajalein atoll, screening was begun in June 1998. Zonal nurses, primary health care staff and voluntary health workers screened the population in the course of house-to-house visits. The Director of Public Health at Ebeye Hospital confirmed the diagnosis, and the coordinator of the TB/Lep Program delivered the chemoprophylaxis to the household contacts in their homes. The program was completed in Kwajalein atoll in September 1998.
Of the 25 inhabited outer islands, nine- Alinglaplap, Jaluit, Arno, Wotje, Ebon, Mololep, Mili, Namdrik and Namu, each with populations of 800 to 2000 and with a high prevalence-were targeted for the program. Teams consisting of the Director of Public Health, the Director of the TB/LEP Program or the WHO STC and two zonal nurses from Majuro are to visit these islands to implement the program in collaboration with the local health staff. During the visits, which will require about two weeks, the team is to screen the population by house-to-house visits, confirm the diagnosis, provide MDT to cases, and administer chemoprophylaxis to the household contacts. By mid-April 1999, only Alinglaplap and Jaluit atolls had been visited. The four nuclear-affected islands-Kili (Bikini), Ennewatak, Ronglap (Meijeto) and Utrik-are the responsibility of the staff of the Health Plan 177, who will conduct the screening, and of the Director of Public Health and Director of the TB/Lep Program, who will confirm the diagnosis. Thus far, a portion of the population of these four islands has been screened. When all of the targeted outer islands have been covered, the program will have included 92 per cent of the population of the country.
According to the projected population for 1998, the population of Majuro and Kwajalein atolls and the two outer islands visited thus far comprises 75 per cent of the population. By April 1999, 91.9 per cent of the population of these four islands had been screened. The atoll-by-atoll data are presented in Table 1.
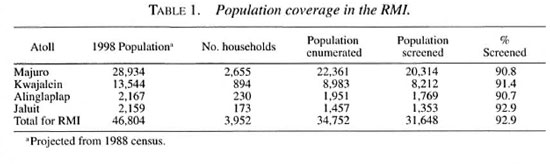
On the four atolls taken together, 144 new cases were detected, yielding a new-case detection rate of 455 per 100,000 population. MB patients represented 31.2 per cent of the total, children under 15 years of age 35.4 per cent, patients presenting with single lesions 46.5 per cent, and those demonstrating WHO grade 2 disability, 2.1 per cent. The atoll-by-atoll data are presented in Table 2.
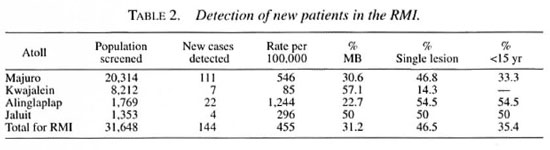
On the four atolls, 305 households included 322 index cases and 3213 contacts. These households constitute 7.7 per cent of the enumerated households, and include 9.2 per cent of the population. Chemoprophylaxis was administered to 2454 (76.4 per cent) of the contacts. Excluding the 382 (12 per cent) contacts who no longer live in these households, 2831 remained for treatment, 86.7 per cent of whom were administered chemoprophylaxis. Treatment was contraindicated in 75 (2.3%), 56 (1.7 per cent) refused treatment, and 246 (7.7 per cent) were absent at the time of the visit.
No adverse reactions to the drugs were reported on Kwajalein, Alinglaplap and Jaluit atolls. On Majuro atoll, five patients complained of adverse reactions-transient nausea, vomiting and dizziness a few hours after the intake or on the following morning.
On the atolls that have been covered thus far, the population enumerated by the screening teams was smaller than that projected for 1998. The projection had been based on the census taken in 1988, which did not take into account emigration, and included those living abroad. It is possible that the actual population is smaller than that projected. On the other hand, the screening teams may have missed some households, and the listing of members of some households may have been incomplete. Therefore, the actual population may lie between the projected and the enumerated figures. For the calculations performed in this paper, the enumerated population has been taken as the actual.
Diagnosis and classification of patients were carried out by the Director of Public Health, the author and, occasionally, by the Director of the TB/Lep Program, all of whom had considerable experience in clinical leprosy. Thus far, the author has encountered only two misdiagnosed cases. For cultural reasons and, occasionally, because of an inability to provide privacy, the buttocks and upper portions of the thighs of many could not be examined. This may have resulted in failure to detect some patients who had only one or a few lesions.
On Majuro atoll, chemoprophylaxis was administered only one to 10 months after screening had been completed, and on Kwajalein atoll, chemoprophylaxis was administered only one to two months after the completion of screening. Because the population of the Marshall Islands is very mobile, 12 per cent of the contacts had already left their households at the time of chemoprophylaxis. On Alinglaplap and Jaluit atolls, there was no time lag between screening and chemoprophylaxis. It had been intended that chemoprophylaxis be administered only under strict supervision; however, that would have required much more time. Therefore, the drugs for some of the contacts, who were absent at the time of the visit were left with a reliable member of the household. It is not known exactly how many of them took the drugs. However, zonal nurses were asked to visit these homes to check the intake of drugs.
During administration of chemoprophylaxis, the definition of household contacts could not be followed strictly. In many instances, members of same family lived in small houses built around a big house. They were originally listed as belonging to different households because they were under different roofs, but when therapy was given to the inhabitants of one of these houses, the rest occasionally claimed that they belonged to the same family and that they have the same risk for exposure, and asked for treatment. Throughout the country, there is also a very high level of movement of people between households of friends and relatives. Under these conditions, a patient may have lived in a number of households within a matter of few years. Likewise, contacts of patients may have moved among different households. Occasionally, some people asked for treatment, saying that they had been contacts, even though they were not living in the household of an index case at the time of the visit. Therefore, the members of some households were administered chemoprophylaxis, although they did not fit the definition of household contacts exactly.
DISCUSSION
Prof. Lechat: Does your definition of an index case include those former patients who have died?
Dr. Kyaw Tin: No.
Dr. Noordeen: What is the risk of disease among household contacts, compared to that in the general population of the RMI?
Prof. Ji: What is the proportion of household contacts among the 144 new cases you detected? This proportion should immediately yield an estimate of the relative risk.
Dr. Kyaw Tin: I can't answer your question at this moment. However, we have the records, and can calculate the risk among contacts, compared to that in the general population.
Dr. Noordeen: In most areas in which leprosy is endemic, the proportion of new cases among household contacts is no greater than 30 to 35 percent. In this situation, to administer chemoprophylaxis only to the contacts suggests that you are attacking only one-third of the problem, and, at best, the chemoprophylaxis can prevent no more than one-third of the cases.
Prof. Levy: Why was the decision taken to limit chemoprophylaxis to household contacts? In the FSM and Kiribati, the chemoprophylaxis was to be administered to entire populations. What led to the decision to restrict chemoprophylaxis in the RMI to contacts?
Dr. Blanc: In this part of the world, cases of leprosy are clustered in foci of high prevalence; this is particularly the case in Micronesia. Although this point has not been studied specifically, it appears that, in these island countries, most cases come from certain localities and certain families.
Dr. Noordeen: I'd like to say something in favor of this approach. Chemoprophylaxis can be administered to contacts at a fraction of the cost of administration to the entire population. Assuming the chemoprophylaxis to be effective, it may appear preferable to prevent a fraction of the cases at low cost rather than to attempt to prevent a larger number of cases at a huge cost.
Prof. Lechat: In fact, we have here three different protocols. In the FSM, virtually the entire population has been administered chemoprophylaxis, whereas in Kiribati, the entire population was screened, but given nothing. In the RMI, they have taken the middle road. The outcome of these three programs will be very interesting.
Dr. Blanc: Prof. Lechat is correct. The three different protocols were deliberately chosen. Chemoprophylaxis can be very costly when administered to the entire population, and confining it to the portion of the population at greatest risk should be much more cost-effective.
Dr. Noordeen: Why was the decision taken not to offer chemoprophylaxis during the first round in Kiribati, as it was in the FSM?
Dr. Blanc: Our first experience with chemoprophylaxis was in the FSM. Here, there was some hesitancy among the decision-makers and medical personnel, but not among the populace.
SUMMARY OF LEPROSY CHEMOPROPHYLAXIS PROGRAMS IN THE WESTERN PACIFIC REGION
Leopold J. Blanc
Western Pacific Regional Office, World Health Organization, Manila, The Philippines.
The programs of the chemoprophylaxis of leprosy already carried out and in progress in the Federated States of Micronesia (FSM), Kiribati and the Republic of the Marshall Islands (RMI) were not designed as research projects. Rather, the primary objective of these programs was to control the disease, and to accelerate achievement of the goal of eliminating leprosy as a public health problem in areas of high prevalence. Hence, it will not be possible to answer many of the important questions that arise. However, we can make use of the available data to derive some conclusions. Although the data are limited, we know from earlier work that chemoprophylaxis is capable of preventing leprosy. That we cannot answer precisely this very important question of efficacy is a source of frustration.
The major conclusions that may be drawn from the combined experience in the three countries are primarily operational. Is such a program feasible? The programs have been very well accepted by the populations of these countries. The frequency of side effects has been minimal, and no serious adverse reactions to ROM have been encountered, despite many thousands of doses.
What degree of completeness of coverage may be expected? During the first round in the FSM, 72 percent of the population was screened, and chemoprophylaxis was administered to 70 percent. In Kiribati, 92 percent of the population has been screened. And in the RMI, 90 percent of the population has been screened, and almost 2500 contacts have been administered chemoprophylaxis.
What may be expected in terms of reduction of the number of new cases detected? From the first to the second round, there was a 75 percent reduction of the number of new cases in the FSM, and a reduction of 85 percent in Kiribati. Eighty new patients were detected during the second round in the FSM, 12 of whom are known to have received chemoprophylaxis during the first round. Of these 12, eight demonstrated only a single lesion, three exhibited PB disease, and only one of the 12 had multibacillary (MB) leprosy.
These programs all suffer from certain limitations. The earlier trials in Pingelap and the South Marquesas Islands also targeted populations in which the prevalence of leprosy was high. In each case, the majority of the local population lived abroad, and efforts were made to administer chemoprophylaxis both to the resident population and to that living off the island. Unlike the trials in Pingelap and the South Marquesas, fully 10 percent of the population of the FSM lives abroad, and it was not possible to treat the emigrant population.
In addition, it is not possible to screen and administer chemoprophylaxis to an entire population in a single day. But because the residents of these islands move about a good deal, it is certainly possible that a resident who received chemoprophylaxis one day may be infected with Mycobacterium leprae as early as the next day, should he go to a village that has not yet been visited by the leprosy team.
A final limitation is that it appears to be necessary to reexamine the concept of household contact. Both in order to assess the attack rate among contacts, and to determine who should be treated, a clear definition of a contact is required, including during what period and for how long a duration there was contact with a patient.
Were these programs worth doing? A first answer is affirmative. In the FSM, there had been, on average, more than 120 new cases annually; during the year following the first round, only 80 new patients were encountered, only 12 of whom are known to have received chemoprophylaxis. Assuming that 120 new cases would have been detected in 1999, these data are consistent with the conclusion that chemoprophylaxis is 90 percent effective.
DISCUSSION
Prof. Smith: Twelve of the 80 new cases had received chemoprophylaxis, implying that the remaining 68 new cases had not. From these data, one may calculate the risk of disease for the individual. In fact, the data suggest that chemoprophylaxis reduced the risk of disease by 92 percent. However, this is different from the objective of the program, which was to stop transmission of M. leprae in the population. The available data don't answer this question.
Prof. Ji: In addition, these data don't discriminate between preventing the disease and merely postponing its appearance.
VACCINE TRIALS IN LEPROSY- VENEZUELA, MALAWI AND INDIA
M. D. Gupte
National Institute of Epidemiology, Chennai, India.
In this paper, I shall review, first, the results of the vaccine trials in Venezuela and Malawi. Then, I shall describe the recently concluded trial in South India, and present the results of the trial. Finally, I shall raise some unresolved issues related to the immunoprophylaxis of leprosy.
In the trial in Venezuela, conducted between 1983 and 1991 ('), the combination of live BCG plus heat-killed Mycobacterium leprae (HKML) was employed to reverse the putative deficiency of the T-cellmediated immune response to M. leprae among the susceptible individuals in the community, the susceptibles being defined by their failure to respond to M. leprae soluble antigen (MLSA) applied as a skin test. Live BCG alone was employed as a Control vaccine. The dose of M. leprae employed was 6 x 10s organisms; two doses of BCG were used-0.2 mg for nonreactors to PPD and 0.04 mg for reactors. The study population consisted of close contacts of 2000 patients-5385 household contacts and 23,728 others of whom 20,376 were nonreactors to MLSA. All were subjected to skin testing with MLSA and PPD before administration of the vaccine, and a 10 percent sample was retested 60 days after vaccination. A new 10 percent random sample was drawn each year for skin testing with MLSA and PPD. Significantly more of those administered the combination vaccine reacted to the Convit skin-test antigen than was the case among those administered BCG alone. Only 39 cases of leprosy were detected in the study population. Twenty of these cases were of particular interest because they had not reacted initially to MLSA; 11 had been administered BCG alone, and nine had been administered the combination vaccine. At least 58 percent protection by previous BCG vaccination, indicated by BCG scars, had been expected based on a case-control study in the trial population. Analysis of the results of this trial indicated that the protective efficacy of BCG was proportional to the number of doses (i.e., the number of BCG scars), and that the protective efficacy of the combination was no greater than that of BCG alone (Table 1).
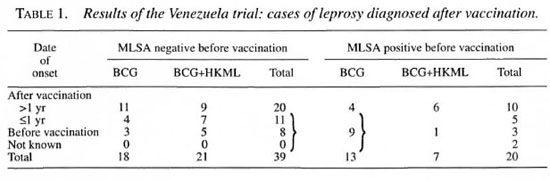
In Malawi, a double-blind trial compared the protective efficacy of a single dose of BCG, of two doses of BCG, and of a single dose of the combination live BCG plus HKML with that of a placebo (3). During the period January 1986 to November 1989, subjects were assigned randomly to one or another vaccine. This trial yielded results similar to those of the trial in Venezuela. Only a small number of cases were detected, despite good evidence that no cases were missed. There were 23 cases among those administered placebo and 12 among those administered BCG, demonstrating that the protective efficacy of BCG was 49 percent. Repeated doses of BCG were more effective than a single initial dose. Addition of HKML to BCG did not enhance the protection obtained by BCG alone. Among children under the age of 15 years, the protective efficacy of BCG with or without HKML was 65 percent (Table 2).

Thus, both the vaccine trial in Venezuela and that in Malawi yielded almost identical conclusions: BCG was effective, and repeated doses of BCG conferred additional protection; the addition to BCG of HKML added virtually nothing to the protection afforded by BCG alone; and the small numbers of new cases detected suggested that almost all of the new cases had been detected.
Two additional points must be considered. In areas in which infection with HIV is prevalent, vaccination with BCG may increase the risk of tuberculosis. And the administration of live BCG to HIV-infectedindividuais also exposes them to the risk of disseminated BCG infection.
In South India, a double-blind, randomized trial was carried out, in which the protective efficacy of BCG, BCG plus HKML,the ICRC bacillus (strain C44), and " Mycobacterium w '" ( M. w ) was measured bycomparison with normal saline as a placebo (2) (Table 3). The study population con-sisted of ali healthy people, ranging in agefrom 1 to 65 years, who resided in 264 contiguous villages adjacent to the city ofChennai (formerly Madras), with a population of 290,000. The two cultivable bacteria-ICRC and M. tv-were administeredin a dose of 109 heat-killed organisms; inthe combination vaccine, live BCG was ad-ministered in a dose of 0.05 mg and HKMLin a dose of 6 x 10` organisms; and BCG was administered alone in a dose of 0.1 mg.As shown in Table 4, 52 percent of the pop-ulation in the age-group 1-4 years demonstrated BCG scars; in the age-group 5-9 years, approximately 20 percent demonstrated scars, as did approximately 11 percent of the population over nine years of age.

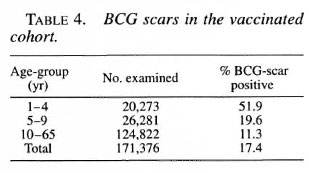
The various vaccines became available at different times during intake, as shown in Table 5. Excluding patients with Ieprosy,children under one year of age, those not present in the study area, and those whowere otherwise ineligible, 74 percent of the total population was eligible for vaccination. Intake, which was carried out betweenJanuary 1991 and July 1993, included 80 percent of the eligible population. The numbers vaccinated were, approximately:ICRC, 22,500; M. tv, 33,700; and placebo,BCG, and BCG plus HKML, 38,300 each.

The first resurvey, which included 86.2 percent of those "vaccinated," was carried out immediately, between August 1993 and February 1995, because of the possibility that missed prevalent leprosy patients mightbe included in the original "vaccinated" cohort. The second resurvey, which included 75.6 percent of those "vaccinated," was carried out between January 1997 and September 1998. Most of the new patients detected in the second resurvey were cases of early paucibacillary (PB) leprosy. Among those administered placebo, the "incidence" of leprosy was 3.2 per 1000 at risk in the first resurvey, and 4.96 per 1000 in the second resurvey. With an incidence of 5 per 1000, employing a one-tailed test (α = 0.05 and β = 0.90), and anticipating a 30 percent loss in the course of the trial, a sample size of 16,000 would be adequate; the sample size of at least 22,500 enrolled in each of the five arms of the trial was thus adequate.
The results of the trial, presented in Table 6, were the following. At the time of the first resurvey, the denominators for the five "vaccines" were nearly identical-approximately 20,000 each. There were 64 cases among those administered the placebo, 82 among those administered BCG, 69 among those administered "BCG plus HKML, M.w -71 cases, and ICRC- 68 cases. Thus, vaccination by all of the vaccines, particularly BCG, appeared to increase susceptibility to M. leprae in both adults and children. However, all of the confidence intervals around the estimates of protection or increased susceptibility included 0; thus, although they were seen in all arms of the trial, none of these "negative effects" was statistically significant.
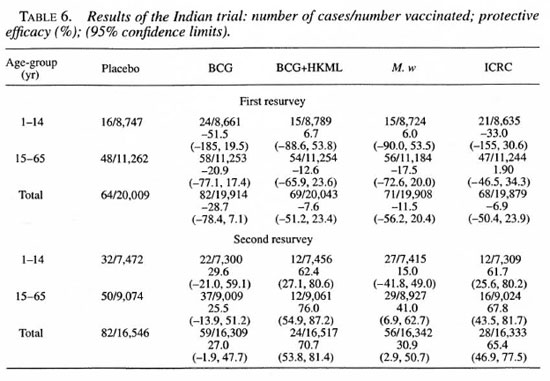
Considering the results of the second resurvey, the denominators for all five arms are again very similar. Among those who had been administered the placebo, 82 new cases were detected-about 1 per 1000 per year, many fewer than expected. Among those administered BCG, there were 59 cases, BCG plus HKML-24, M. w- 56, and ICRC-28. The protective efficacy of BCG was 27 percent (not significantly different from 0), of BCG plus HKML 71 percent, of M. w 31 percent, and of ICRC 65 percent. Thus, BCG plus HKML and ICRC were almost equally effective, as were BCG and M. w. In general, the various vaccines demonstrated approximately similar efficacy in children and adults. Assuming that, in this highly endemic population, virtually all the adult population had been infected with M. leprae, all of the vaccines appeared protective even in those already infected. BCG and M. w afforded approximately the same level of protection; whereas both BCG plus HKML and the ICRC bacillus afforded a higher degree of protection.
The study population was observed for two months after vaccination to detect any vaccination-related side effects. Among those individuals administered the ICRC vaccine, 71 (approximately 0.2 percent) suffered fluctuant adenitis. Acid-fast bacilli could not be demonstrated in any of the discharges (Table 7).
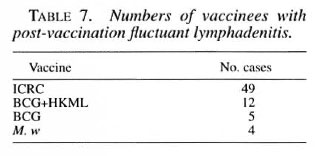
Thus, the combination vaccine, BCG plus HKML, and the ICRC vaccine appeared to be potentially useful immunoprophylactic agents, although the results of the trial in South India differ from those of the trials in Venezuela and Malawi.
Three issues remain to be addressed: 1) the usefulness of BCG in countries in which it has been found to be effective; 2) the possibility of using a vaccine that includes M. leprae; and 3) the usefulness of the ICRC vaccine in India and in other countries in which the epidemiologic pattern of the disease is similar to that in India.
Although earlier studies carried out in Uganda and New Guinea had demonstrated that BCG is an effective protective vaccine against leprosy, the results of the two vaccine studies conducted in South India-the current trial and that carried out earlier in a study of the protective efficacy of BCG in both tuberculosis and leprosy-show that BCG can have only a very limited role in the prevention of leprosy in India. Unfortunately, in the countries in which BCG can be of use, HIV is highly endemic, precluding the use of live BCG in these communities. Thus, in the context of the present day, one could hardly consider BCG for prevention of leprosy.
With respect to vaccines that contain M. leprae, it must be recognized that armadillo-derived M. leprae will not be available for large-scale programs of vaccination against leprosy. Because the combination BCG plus HKML has been found effective in India, there may be a place for recombinant BCG containing an appropriate part of the M. leprae genome. However, new trials of such a leprosy vaccine will be almost impossible to mount. Thus, in the absence of surrogate markers for immunity against leprosy, M. leprae-based vaccines cannot be tested.
The ICRC bacillus, isolated by Khanolkar, Ranadive and Bapat in the 1950s from patients with lepromatous leprosy, is a cultivable Mycobacterium. The vaccine prepared from this organism has been found to be useful in both adults and children, suggesting that it could be useful even in infected individuals. Moreover, the efficacy of this vaccine has been found to be similar in both those found to have BCG scars and those without scars. Although there has been little interest in this vaccine outside India, there is an immediate need to develop this vaccine and subject it to Phase IV studies. Thus, if there exists a need for a vaccine against leprosy, serious thought should be given to a vaccine prepared from the ICRC bacillus.
DISCUSSION
Prof. Ji: I must congratulate Dr. Gupte and his colleagues for having planned and conducted a truly excellent trial. I have three questions. First, is it necessary to mount a second trial of the ICRC vaccine in a country other than India? Second, of the 71 patients who developed fluctuant adenitis, 51, a disproportionately large number, had been administered the ICRC vaccine. Is this an acceptable risk? And has this phenomenon been explained? Finally, what is the nature of the ICRC bacillus. We know that it is cultivable. Is it a single species, or is it in fact a complex? Has its taxonomy been studied in terms of its genome?
Dr. Gupte: I don't think that a second formal trial of the ICRC vaccine, which represents a Phase III study, is needed. Phase IV studies, such as the chemoprophylaxis program carried out here in the FSM, need not be carried out by a double-blind, randomized, controlled design; these should be carried out in other countries.
With respect to the side effects, vaccination with a potent preparation of BCG frequently gives rise to suppurative adenitis among 2 to 3 percent of those vaccinated; whereas we encountered a frequency of only 0.2 to 0.25 percent. One must balance the protective efficacy against the risk of side effects.
The question of the nature of the ICRC bacillus has been raised repeatedly. The organism belongs to the M. avium-intracellulare complex. However, a detailed characterization of the organism is certainly called for, and we are presently considering arrangements for such work.
Dr. Blanc: I have a question with respect to BCG. What is the explanation of the failure of BCG to protect against both leprosy and tuberculosis in India, whereas trials in Africa have demonstrated good protection against leprosy, and relatively good protection against tuberculosis?
Dr. Gupte: Although several hypotheses have been advanced, we simply don't know why BCG has failed in India, whereas it has been effective in Africa.
Dr. Diletto: What is the explanation for the negative effect of all of the vaccines seen in the first resurvey? Second, the policy of WHO with respect to BCG and HIV is to administer the vaccine to everyone who does not have clinical AIDS. If disseminated BCG infection were a problem among the HIV-infected, surely this policy would have been modified.
Dr. Gupte: Certainly, HIV positivity is not a contraindication to BCG vaccination, even in Africa. Both in Malawi and in India, BCG vaccination appeared to precipitate tuberculosis. Many in Africa who are infected with HIV are also infected with M. tuberculosis. It appears undesirable to administer BCG to an HIV-infected individual to prevent leprosy and, instead, to precipitate clinical tuberculosis.
Dr. Noordeen: The negative effect seen in the first resurvey is interesting. It appears likely that at least some of the new cases detected in the first survey had been missed in the survey carried out before the vaccines were administered. If one considers the combined results of the two resurveys, one is struck by an apparent paradox. The vaccination appears both to precipitate clinical disease among those incubating the infection, as shown by the first resurvey, at the same time that it prevents ciinical disease among those incubating the infection, as shown by the second resurvey.
Dr. Gupte: The negative effect of the vaccines was not on account of missed cases. Were missed cases a factor, the negative effect would have been considerably greater than that we encountered.
Dr. Izumi: Is the ICRC vaccine killed? Second, to which species of Mycobacterium does the bacillus belong? Third, has the ICRC bacillus been studied in some mouse system, in which production of interferon-γ or changes of cytokine profiles have been studied? Finally, how can the differences of efficacy according to age group be explained?
Dr. Gupte: Both M. w and ICRC were killed vaccines; M. w was autoclaved and ICRC was irradiated. With respect to surrogate measures of protection, we carried out Phase II studies of all vaccine candidates, which included skin testing with Mitsuda antigen and with Rees' soluble antigen. After vaccination with the combination BCG plus HKML, there was a high frequency of induration, whereas, after administration of M. w, no reactions were observed. After vaccination with ICRC, induration was observed in response to skin testing, but it was not as prominent as that after BCG plus HKML. We have not examined any other measures of the immune response.
REFERENCES
1. CONVIT, J., SAMPSON, C, ZUNIGA, M., ET AL.. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: Preliminary results. Lancet 339 (1992) 446-450.
2. GUPTE, M. D., VALLISHAYKE, R. S., ANANTHARAMAN, D. S., ET AL. Comparative leprosy vaccine trial in South India. Indian J. Lepr. 70 (1998) 369-388.
3. KARONGA PREVENTION TRIAL GROUP. Randomized controlled trial of single BCG, repeated BCG or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348 (1996) 17-24.
PREVENTIVE TREATMENT OF LEPROSY: NEEDS, OPPORTUNITIES, AND FEASIBILITY
W. C. S. Smith; C. M. Smith
Department of Public Health, Medical School, University of Aberdeen, Aberdeen, Scotland.
That the rates at which new cases of leprosy are detected have failed to decrease suggests that we should review the current approaches to leprosy control, and consider in particular whether there are justifiable arguments to explore preventive approaches. There are complex reasons for the continuing high levels of global new-case detection rates. What is not clear is whether the trends in case detection reflect underlying secular trends in transmission. This is the first issue to be addressed in this review.
Having considered the case for preventive strategies, the second issue to be addressed is what potential approaches are available. The evidence of effectiveness of the available approaches should be systematically reviewed. These may be single approaches or combinations of methods, which together could confer protection. It is also possible that other interventions would be effective but have not yet been tested in the field. These should be reviewed and recommendations made regarding the value of such research.
The final stage in this analysis is to consider the feasibility of implementing these preventive strategies. Practical and ethical issues must considered, the groups to be treated must be identified, and the magnitude of the effect if such preventive approaches were implemented should be estimated.
NEED FOR PREVENTIVE STRATEGIES
The global number of new cases of leprosy detected each year for the last ten years has remained relatively static; indeed, there has been an increase over the last few years (The Figure). However, this global trend masks a diverse picture at the national level; some countries (34) show increasing numbers of new cases, others show decreases, and some show considerable variation. It is clear that case detection does not directly reflect the true incidence of disease or current transmission of Mycobacterium leprae (7, 15, 30). One of the expectations of the elimination strategy was that extensive use of multidrug therapy (MDT) would reduce the quantum of infection in the community, leading in time to a reduction of transmission. However, although administration of MDT is thought to block transmission almost immediately, it is more than likely that transmission occurs prior to detection and treatment. Recent developments using polymerase chain reaction (PCR) technology have suggested the possibilities that there may be asymptomatic carriage (7) and environmental reservoirs of M. leprae. However, pre-treatment transmission and the long incubation period are probably sufficient to explain the lack of an immediate effect of MDT on new-case detection rates. Moreover, it is well accepted that leprosy cases do not occur randomly in the community, but rather in clusters.
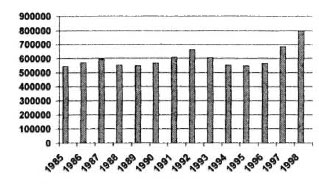
The figure. Global trends in new-case detection.
Operational factors profoundly affect case detection (l8), and are likely to be responsible for the sustained high levels of case detection and the recent increases of the rates at which new cases are detected in some countries. The intensity of case detection activities influences case-detection rates, as does the "earliness" of case detection in the disease process (8). Geographical coverage has increased as the leprosy elimination program has been implemented. Initiatives such as the leprosy elimination campaigns have increased new-case detection in several countries (35), partly through finding patients who have had the disease for a long time (so-called back-log cases), and also by re-registering patients who had defaulted from treatment in the past. The quality of leprosy information systems has also improved; this would lead to an apparent increase of case numbers.
The occurrence of disease is the result of a balance among the agent, the environment and the host. Most attention in the leprosy elimination program has been paid to attacking the organism by means of MDT; however, important changes have also occurred in the environment and in the host. Socioeconomic improvements such as better nutrition, better sanitation, improved housing, and less overcrowding are likely to contribute to changes of the epidemiologic pattern. The widespread use of BCG (12) is likely to have been responsible for increased host immunity to M. leprae.
Disease modeling is a scientific approach that has been used to understand the epidemiology of leprosy and predict future trends. This approach is useful because, among other things, it identifies the gaps in our understanding with respect to transmission of the organism. These models demonstrate that chemotherapy has an effect, but because MDT was implemented immediately after dapsone monotherapy, it is impossible to detect a difference. On the other hand, they predict that if chemotherapy were withdrawn, the incidence would increase. Although the output of these models must be interpreted with caution, they suggest that trends in leprosy incidence are likely to be gradual, and it is not realistic to expect large changes of disease incidence in the course of a few years. These observations are not surprising, in view of the long incubation period of leprosy and the slowness of change of the disease process.
The current situation is confused where the underlying incidence of disease is uncertain, and the reduction of transmission of M. leprae is probably a gradual process. It appears reasonable, therefore, that the potential of strategies to reduce transmission and prevent disease be explored.
THE OPPORTUNITY: CHEMOPROPHYLAXIS AND IMMUNOPROPHYLAXIS
Chemoprophylaxis. A number of chemoprophylaxis trials using dapsone were conducted prior to the introduction of MDT (10, 22, 24, 27, 28) progressive emergence of dapsone resistance, followed by implementation of MDT, changed research priorities. Attention was paid to implementation of the MDT program, with the prospect that MDT would prove to be the tool that would interrupt transmission by reducing the community burden of infection. However, the findings of these trials are interesting and relevant to a consideration of methods to prevent lepros y.
The evidence of efficacy of chemoprophylaxis demonstrated by trials, including unpublished work, has been systematically reviewed and critically appraised (29). Fourteen trials, most employing dapsone, acedapsone or rifampin, were identified. Randomized controlled trials demonstrated 54 percent protective efficacy, whereas nonrandomized trials showed 72 percent protection. In the trials that involved household contacts, the "numbers necessary to treat" (NNT) in order to prevent one new case were small, ranging from 9 to 63, whereas the NNT for trials that involved entire communities were much larger, ranging from 120 to 393 (The Table). The difference of the NNT between household contacts and the entire community reflects the difference of absolute risk of leprosy rather than differences of protective efficacy. There is a tendency for the effectiveness of chemoprophylaxis to wane over time. It can be concluded that dapsone chemoprophylaxis is effective, with an overall protective efficacy of about 60 percent. Newer drugs such as rifampin, minocycline and ofloxacin are currently under investigation, and formal randomized controlled trials are now needed. These offer a potential strategy for the prevention of leprosy in "high-risk" groups. The use of chemoprophylaxis in "low-risk" groups would result in very large NNT, even if the protective efficacy were very good.
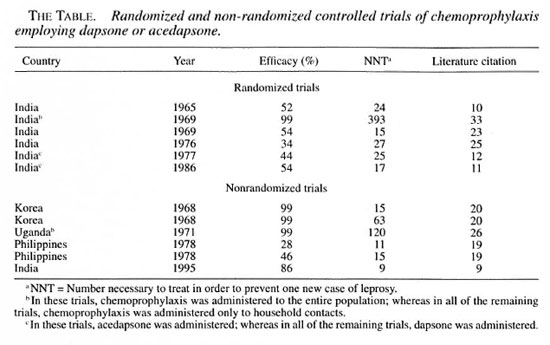
Immunoprophylaxis. The effectiveness of BCG and BCG plus heat-killed M. leprae (HKML) has been assessed in trials in a number of countries, including Uganda, Burma, Papua New Guinea, Malawi, India and Venezuela (6, 11, 12, 21). Although there is some country-to-country variation, the results of these trials consistently show BCG to have a protective efficacy about 50 percent. There is also evidence that a repeated dose of BCG can confer a further 50 percent protection. More recently, results from a trial in South India have shown that BCG plus HKML can be effective, as can the ICRC bacillus, whereas the protective effect of BCG alone and of Mycobacterium w was smaller (14). This trial also showed a negative effect of all vaccines at the initial follow up, but a longer-term protective effect.
This considerable body of evidence demonstrates that there are available today effective vaccines against leprosy but, again, the NNT is large, unless the vaccines are applied to high-risk groups. Paradoxically, the repeated use of BCG is reported to be of little benefit in protection against tuberculosis, and there may also be practical difficulties with the use of repeated BCG in areas in which HIV infection is prevalent.
Chemoprophylaxis and immunoprophylaxis.That the protective efficacy of chemoprophylaxis is approximately 60 percent and that of immunoprophylaxis at least 50 percent raises the question of whether there would be an added effect if both approaches were used together. Their activity could be additive, because their modes of action are different. Chemoprophylaxis would be effective in subclinical infection and during the incubation period and, perhaps, in asymptomatic carriers. Immunoprophylaxis, on the other hand, would increase host resistance to infection and provide protection against future exposure. It could be argued that the initial adverse effect of vaccines could be controlled by chemotherapy in individuals who are presumably incubating the infection at the time of administration.
Clearly, chemotherapy should not be administered at the same time as BCG because this would kill the live organisms, but it could be administered 2 or 3 days earlier. This restriction however would not apply to vaccines consisting of HKML or the ICRC bacillus, which employ killed organisms.
There are precedents for combining chemoprophylaxis and immunoprophylaxis in the prevention and control of other infectious diseases. For example, this approach is recommended for household contacts of meningococcal infection in a number of countries in which infection is caused by serotype A or C (1,2, 4). This targeted approach attempts to deal with the problem of asymptomatic carriers and those incubating the disease. However, mass immunization alone is generally not recommended as a disease control measure at present (5). The combination of chemoprophylaxis and vaccine has also been recommended in the control of tuberculosis, malaria and in influenza epidemics in high-risk groups (3, 16, 32).
Other uses of combined chemoprophylaxis and immunoprophylaxis are among asplenic patients, patients with leukemia, and after liver transplant. Chemoprophylaxis may be recommended for those at risk in whom vaccination is contraindicated, particularly when live vaccines are to be used.
FEASIBILITY OF PREVENTION
Prevention of disease using chemoprophylaxis or immunoprophylaxis can be considered in communities at high risk of disease, or in identifiable subgroups or communities within endemic countries. However, the justification for such an approach will depend on the level of risk, and a number of other issues must be considered, including costs, perceived risk and political interests. Leprosy cases tend to be clustered geographically and such clusters, based in villages or defined communities, may be identified as being at particularly high risk.
Household contacts are a readily definable group recognized to be at increased risk. The definition of a household contact may vary among communities, and broader definitions, which include adjacent households, may be appropriate (l7). Other close-contact exposures outside the household, such as in the workplace and at school, may also be considered, although there is little evidence that these groups are at high risk of leprosy.
Household contacts are at relatively higher risk than the rest of the community. The degree of risk varies by the classification of the index cases- i.e., the risk is higher among contacts of multibacillary (MB) than to those of paucibacillary (PB) patients. The relative risk, compared to the rest of the country, can be very high (over 200) in countries with low endemicity (31). However, the difference of risk is more crucial than the relative risk, as is discussed below. In some countries, 20-30 percent of new cases are estimated to be household contacts; targeting this group would have the effect of reducing disease incidence at a national level.
The specific aspects of feasibility which should be considered are: logistics, acceptability and impact.
Logistics. The most opportune time to intervene with prophylaxis for household contacts is at the point of case detection or, for community interventions, when there is a high level of awareness and perception of risk. For household contacts, the time of registering the index case would be most opportune; the household would be aware of the risk, and the household contacts are likely to be examined and provided with information about the disease at this time. This depends, however, on the diagnosis of leprosy being made in proximity to the household, which may not always be the case, and also on all members of the household being available at that time. Proxy distribution of the treatment by the head of the household or the index case may be an option.
Chemoprophylaxis may be more feasible than immunoprophylaxis, especially if the drugs used for chemoprophylaxis weie also those used for treatment. Use of a vaccine introduces more complex issues, such as cold-chain equipment, needles, syringes and their safe disposal, all of which have cost implications. The timing of the administration of chemoprophylaxis and vaccines may need to be considered if one is likely to interfere with the other; for example, a live vaccine could be inactivated by the chemoprophylaxis.
Acceptability. The acceptability of these preventive strategies to the individual household and the community must also be considered. This may vary from country to country, and would be greatly influenced by the information provided. Chemoprophylaxis or immunoprophylaxis could be highly acceptable as a means of providing perceived protection at a time of acute awareness of risk within a household or community. Chemoprophylaxis may also have a beneficial effect on compliance with treatment by the index case, and contribute to allaying anxiety. On the other hand, there are issues of confidentiality to be considered; the index case may not wish the diagnosis to be revealed to the members of his household. Treatment of the household may increase stigma locally and lead to increased concerns about the risk of disease.
Impact. The impact of prophylaxis is dependent in part on the degree of protective efficacy, and is likely to be about 50 percent for either immunoprophylaxis or chemoprophylaxis alone. The protective efficacy of both in combination is at present a matter for conjecture. However, the impact depends also on the level of risk- i.e., the absolute risk, compared to that in the general population. A low absolute risk would render the impact of the intervention small.
The impact can be estimated for an individual at high risk or for the community as a whole. It is possible to have a sizeable effect on one but not the other, both being legitimate objectives. The impact on the individual can be derived from the NNT, which would be equivalent to the difference of risk. This number has been estimated to be quite small in chemoprophylaxis- trials among household contacts. The impact on the community depends on the proportion of new cases arising from household contacts of existing cases (29). The other factor in assessing the likely impact on communities is the number of household contacts per index case; this could vary widely between communities. Annual incidence rates in some highly endemic countries can be of the order of 1 per 1000. The application of a prophylaxis which is 50 percent effective would result in an NNT of 2000; clearly, much higher levels of risk are required to achieve a smaller NNT. If the prophylaxis were applied to all household contacts in an area in which 30 percent of new cases arise from household contacts (13), the estimated reduction of incidence at community level could be about 15 percent.
CONCLUSIONS
There is evidence that both chemoprophylaxis and immunoprophylaxis are effective in the prevention of leprosy. Most of the evidence with respect to chemoprophylaxis is based on trials of dapsone or acedapsone; therefore, randomized controlled trials using other drugs such as rifampin are needed. The likely effectiveness of combined chemo- and immunoprophylaxis also requires further exploration. The theoretical basis of such a combined approach may justify a trial.
Important feasibility issues need to be considered-particularly logistics, acceptability and the estimated impact of the intervention relative to cost. The impact will vary among communities and subgroups, depending on the levels of risk and the proportion of the community identified as being at high risk. The NNT is one method which could be used in assessing the costs relative to the estimated impact of the intervention. There is also a need to identify groups at high risk in which the benefits would justify the cost.
Finally, the continuing high levels of case detection globally indicate that prevention is an area worth pursuing in terms of new research initiatives.
DISCUSSION
Dr. Ferrugia: Speaking of cost-effectiveness, who among us has the slightest idea of the cost of one dose of ROM, and how costly is the chemoprophylaxis campaign?
Dr. Noordeen: There is no difficulty in calculating the cost of the drug. A single dose of ROM now costs about US$3. The cost of the campaign is another matter; its cost will vary with the local situation.
Prof. Smith: I was thinking in more general terms. The larger the number of people to whom you must administer the prophylaxis to prevent one case, the less cost-effective the campaign becomes. To treat five or 10 household contacts by an effective chemoprophylaxis to prevent one case appears much less expensive than to administer BCG to 5000 people to prevent one case.
Dr. Izumi: In Indonesia, the endemicity varies widely from province to province. For example, the southern Celebes Islands are highly endemic, whereas the eastern Celebes are an area of low endemicity, even though the socioeconomic conditions are very similar. Therefore, we could imagine different strategies for different areas, and should tailor the preventive methods to fit the endemicity of the local situation.
Prof. Smith: I agree. We should ask ourselves how high must the absolute level of endemicity be in order to justify administration of chemoprophylaxis to an entire population. On the other hand, administration of chemoprophylaxis only to household contacts is an approach that is justified in the areas of lowest endemicity.
Dr. Noordeen: You are suggesting that household contacts in areas of low endemicity are at the same level of risk as those in areas of high endemicity. However, I am aware of at least one study in which it was demonstrated that the risk to contacts was proportional to the endemicity of the area in which they resided. Therefore, the NNT would be greater in areas of low endemicity than in areas of high endemicity.
Dr. Gupte: Even in the highly endemic area of the leprosy vaccine trial, the risk of disease among contacts was greater than that in the general population. As the level of endemicity increases, so does the risk of disease among contacts. At the same time, in two areas of low endemicity in Tamil Nadhu, new cases are detected only among contacts and those who had travelled to endemic areas and returned.
Dr. Noordeen: Dr. Convit's vaccine trial in Venezuela yielded some very interesting data. Most of the new cases were detected among those not included in the group of household contacts and the larger number of nonhousehold contacts, this despite the fact that Venezuela represents a situation of relatively low endemicity.
Dr. Diletto: The level of endemicity in a country does not reflect the reality, especially in the post-elimination phase. In the FSM, which is an area of high endemicity, the new cases are not randomly distributed. And in countries of low endemicity, there are pockets of high endemicity. Prophylactic measures should be reserved for these pockets.
Acknowledgment. The authors wish to acknowledge the assistance, advice and comments on drafts of this paper received from the members of the MILEP2 Study Group. The MILEP2 (Mucosal Immunology of Leprosy) Study Group is funded by the European Commission under the Fourth R&D program "Scientific and Technological Co-operation with Developing Countries (INCODC)."
REFERENCES
1. ANONYMOUS. Meningococcal disease prevention and control strategies for practice based physicians. Ped. 97 (1996) 404-411.
2. ANONYMOUS. Control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly. Rep. 46 (1997) 13-21.
3. ANONYMOUS. The control of tuberculosis in Scotland: TB Guidelines. The Scottish Office Department of Health CH404 (1998) 34.
4. BEATY, H.N. Rifampicin and minocycline in meningococcal disease. Rev. Infect. Dis. 5 Suppl. 3 (1983) S451-S458.
5. BJUNE, G., HOIBY, E.A. and GRONNESBY, J.K. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338(1991) 1093-1096.
6. BAGSHAWE, A., SCOTT, G.C., RUSSELL, D.A., WIGLEY, S.C., MERIANOS, A., and BERRY, G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea, 1963-79 . Bull. WHO 67 (1989) 389-399.
7. CREE, I.A. and SMITH, W.C. Leprosy transmission and mucosal immunity: towards eradication? Lepr. Rev 69(1998) 112-121.
8. DAY, R., LEVER, P. and ASRI, M. Leprosy control in 7 districts of south Sulawesi, Indonesia, 1986-91 . Lepr. Rev. 63 (1992) 247-254.
9. DAYAL, R. and BHARADWAJ, V.P. Prevention and early detection of leprosy in children. J. Trop. Pediatrics 41 (1995) 132-138.
10. DHARMENDRA. Prophylactic value of DDS against leprosy: An interim report. Lepr. India 37 (1965 ) 1-20.
11. FINE, P.E. BCG vaccination against tuberculosis and leprosy. Br. Med. Bull. 44 (1988) 691-703.
12. FINE, P.E. and SMITH, P.G. Vaccination Against Leprosy-The View from 1996. Lepr. Rev. 67 (1996)249-252.
13. FINE, P.E., STERNE, J.A., PONNIGHAUS, J.M., ETAL. Household and dwelling contact as risk factors for leprosy in northern Malawi. Am. J. Epidemiol. 146(1997)91-102.
14. GUPTE, M.D. VALLISHAYEE, R.S., ANANTHARAMAN, D.S. and NAGARAJU, B. Comparative leprosy vaccine trial in South India. Ind. J. Lepr. 70 (1998 ) 369-387.
15. JAKEMAN, P. and SMITH, W.C. Evaluation of a multidrug therapy programme of leprosy control. Lepr. Rev. 65 (1994) 289-296.
16. KENNEDY, M.M. Influenza viral infections, presentation, prevention and treatment. Nurse Pract. 23 (1998)21-39.
17. KLATSER, P.R., VAN BEERS, S., MADJID, B., DAY, R. and DE WIT, M.Y. Detection of Mycobacterium leprae nasal carriers in populations in which leprosy is endemic. J. Clin. Microbiol. 31 (1993 ) 2947-2951.
18. KRISHNAMURTHY, P., RAO, P.S. and SUBRAMANIAN, M. The influence of operational factors in the profile of monolesional leprosy cases in South India. Lepr. Rev. 65(1994) 130-136.
19. LARA, C.B. DDS Chemoprophylaxis for leprosy in children at Culion Sanitarium Palawan, Philippines. Acta Leprol. 70 (1978) 35-58.
20. LEW, J. and KIM, Y.S. Chemoprophylaxis of leprosy contacts with dapsone. int. J. Lepr. 36 (1968)
21. LWIN, K. BCG Vaccination of children against leprosy: fourteen-year findings of the trial in Burma. Bull. WHO 63 (1985) 1069-1075.
22. NEELAN, P.N. AND SIRUMIÎAN, P. Limited duration acedapsone prophylaxis in leprosy. Indian J Lepr. 58(1986) 251-256.
23. NOORDEEN, S.K. Chemoprophylaxis in leprosy. Lepr. India 41 (1969) 247-254.
24. NOORDKEN, S.K. Long term effects of chemoprophylaxis among contacts of lepromatous cases. results of 8 1/2 years follow-up. Lepr. India 49 (1977) 504-509.
25. NOORDLHN, S.K. and NEELAN, P.N. Chemophrophylaxis among contacts of non- lepromatous leprosy. Lepr. India 48 (1976) 635-642.
26. OISYUI.A, Y., IBWORO, C. and CHUM, H.J. Four years experience with dapsone as prophylaxis against leprosy. Lepr. Rev. 42 (1971) 98-100.
27. RUSSELL, D.A., WORTH, R.M., JANO, B., FASAL, P. and SHEPARD, C.C. Acedapsone in the prevention of leprosy. Am. J. Trop. Med. Hyg. 28 (1979) 559-563.
28. SLOAN, N.R. and WORTH, R.M. ACEDAPSONE IN LEPROSY CHEMOPROPHYLAXIS: Field trial in three high prevalence villages of micronesia. Int. J. Lepr. 40(1972)40-47.
29. SMITH, CM. and SMITH, W.C.S. Chemoprophylaxis is effective in the prevention of leprosy in endemic countries. Submitted for publication (1999).
30. SMITH W.C.S . We need to know what is happening to the incidence of leprosy. Lepr. Rev. 68 (1997) 195-200.
31. STLS, P. and MALATRL, X. Will the leprosy endemic in rwanda soon be under control? Lepr. Rev. 60(1989) 139-146.
32. TRAPK, J.F. and ROGIER, C. Combating malaria morbidity and mortality by reducing transmission. Parasitol. Today 12 (1996) 237-240.
33. WARDLKAR, R.V. DDS Prophylaxis against leprosy. Lepr. India 39 (1967) 155-159.
34. WORLD HEALTH ORGANIZATION. Trends in leprosy detection. Weekly Epidemiological Record 73 (1998) 169-176.
35. WORLD HEALTH ORGANISATION. Leprosy elimination campaigns (LEC's). Weekly Epidemiological Record 73 (1998) 177-184.
DRUGS AND REGIMENS FOR PREVENTIVE THERAPY AGAINST TUBERCULOSIS, DISSEMINATED MYCOBACTERIUM AVIUM COMPLEX INFECTION AND LEPROSY
Baohong Ji, M.D., Jacques H. Grosset, M.D.
Faculté de Médecine Pitie-Salpetriere, Paris, France.
Preventive therapy, or chemoprophylaxis, refers to the administration of effective drugs to an individual with confirmed or suspected latent, or subclinical, infection, aiming to prevent development of overt disease. In theory, when a large number of people with latent infection exist in the community, massive application of chemoprophylaxis would lead to a rapid decline of the occurrence of the disease. However, a number of operational and technical constraints have limited its application in the control of infectious diseases; with the exception of yaws, experience with mass chemoprophylaxis for disease control has not been encouraging (41). On the other hand, significant progress has been made during the last decade in identifying effective drugs for chemoprophylaxis against infection with Mycobacterium tuberculosis and M. avium complex (MAC), and research on chemoprophylaxis against M. leprae would benefit from what has been learned with regard to chemoprophylaxis against these two infections.
In choosing an appropriate regimen for chemoprophylaxis, one should consider not only efficacy, but also a number of other elements, including tolerability, cost and the potential of drug-drug interaction. Unfortunately, a highly effective, nontoxic, low-cost and easily applicable drug for the prevention of any of the following three diseases has yet to be identified.
TUBERCULOSIS
Chemoprophylaxis with isoniazid (INH)
Experiments have clearly demonstrated that administration of INH was capable of preventing tuberculosis in guinea pigs, whether chemoprophylaxis was administered daily or every few days, as long as drug administration lasted between six and 12 months (l0,45). Administration for less than six months was associated with a high relapse rate, whereas administration for longer than one year conferred no additional benefit. Subsequently, a large number of randomized, placebo-controlled, clinical trials, involving more than 100,000 participants at risk of tuberculosis, confirmed the protective effect of INH observed in guinea pigs C (13,19). The effectiveness of chemoprophylaxis, in terms of reduction of the incidence of tuberculosis among those administered INH compared with that among those administered placebo, varied from 25 to 92 percent. The optimal duration of therapy was six to 12 months; administration for longer than 12 months was not associated with increased protection.
INH chemoprophylaxis is recommended by the American Thoracic Society and the U. S. Centers for Disease Control and Prevention (1) for all tuberculin-positive individuals at high risk, especially those infected with HIV and children who are close contacts of tuberculosis patients, and for some tuberculin-negative individuals, such as children who are close contacts of infectious cases and HIV-infected persons. INH is administered daily to both adults and children in a dosage of 5 mg per kg body weight, to a maximum of 300 mg. When supervised therapy is required, INH may be administered twice weekly in a dosage of 15 mg per kg, to a maximum of 900 mg. The duration of treatment is at least six months, and 12 months are required for those infected with HIV.
However, INH chemoprophylaxis is not strikingly effective, conferring no more than 70 percent protection (2,10). In addition, it is difticult for people who are otherwise healthy and asymptomatic to take medication for six to 12 months to prevent an illness that may not occur in the absence of chemoprophylaxis, and is readily treatable should it occur. Consequently, noncompliance with INH chemoprophylaxis is common, substantially reducing its efficacy (55). In addition, it is known that, in the course of chemoprophylaxis, INH causes toxic hepatitis in approximately 1 percent (35), and death in fewer than 0.1 percent (35,56) of those to whom it is administered. As a result, healthy asymptomatic people may be unwilling to accept this risk (2,55). Finally, INH is inactive against INH-resistant strains of M. tuberculosis, which are common in both developed and developing countries. Therefore, an alternative regimen of shorter duration, employing safe and more effective drugs, is needed.
"Short-course" chemoprophylaxis with rifamycins
Rifampin. Rifampin (RMP) is far more bactericidal than is INH. In mice harboring small and stable populations of M. tuberculosis (8, 28, 36) a situation that mimics the bacterial population of latent M. tuberculosis infection of man, we demonstrated that the administration of RMP plus pyrazinamide (PZA) for two months, or RMP alone for three months was more effective than INH alone for six months. These results suggested that the use of RMP in the chemoprophylaxis of tuberculosis may permit dramatic shortening of the duration of treatment (8,28,36). An increasing number of clinical studies have confirmed that RMP, alone or in combination with other drugs, may be effective in chemoprophylaxis. A trial among patients with silicosis revealed RMP administered alone for 12 weeks conferred at least the same level of protection as INH administered alone for 24 weeks, and that RMP monotherapy was less hepatotoxic than INH-containing regimens (22). In another trial, 157 adolescents probably infected with strains of M. tuberculosis resistant to INH were administered chemoprophylaxis with RMP in a daily dosage of 10 mg per kg for 12 weeks (57); none of the adolescents developed tuberculosis, and the protective efficacy was calculated to be at least 56 percent. A study in Uganda demonstrated that daily administration of RMPINH or RMP-INH-PZA for three months conferred significant protection compared with placebo treatment, although neither regimen was superior to INH (58).
"Short-course" chemoprophylaxis with RMP-containing regimens may improve compliance, but cannot totally overcome the problem, unless the drugs are administered under supervision. However, it is not feasible to supervise daily treatment in routine practice, and the activity of RMP against M. tuberculosis decreases tremendously when it is administered intermittently, even thrice weekly, a consequence of its pharmacokinetic properties (28). Therefore, we have continued our efforts to identify antimicrobials with bactericidal activity at least equal to that of RMP but suitable for intermittent administration.
Rifapentine. We compared the pharmacokinetics and anti-M. tuberculosis activity of RMP with those of two other rifamycin derivatives-rifapentine (RPT) and rifabutin (RBT) (28). After a single dose of 10 mg per kg body weight, RPT exhibited the highest serum peak level (Cmax) and the longest half-life (t1/2), and RBT the lowest Cmax and the shortest t1/2. On a weight-to-weight basis, both RPT and RBT were more bactericidal than RMP; RBT appeared to be the most rapidly bactericidal. After administration for six weeks, the bactericidal activity of RBT was comparable to that of RMP and RPT administered in the same daily dosage for 12 weeks. Upon intermittent administration, however, RPT was the most active and RMP the least active. The bactericidal activity of RPT administered twice weekly for 12 weeks in a dosage of 10 mg per kg was comparable to that of RBT administered daily for six weeks or that of RMP administered daily for 12 weeks in the same dosage. Furthermore, the bactericidal activity of RPT was retained even when the drug was administered once weekly or once every 2 weeks; 90-99 percent bactericide was observed after six weeks, and greater than 99.9 percent bactericide after 12 weeks, comparable to the bactericidal effect of RBT administered twice weekly or RMP administered thrice weekly.
That RPT is a promising candidate for the short-course, supervisable chemoprophylaxis of tuberculosis was confirmed in a series of experiments conducted in immunocompetent (normal) mice, representing HIV-negative hosts, and in congenitally athymic (nude) mice, which mimic to a certain extent patients with AIDS (8-11-20). The activity of RPT was found to be significantly enhanced when INH was added at the same dosing frequency; the combination of RPT-INH administered once weekly for six months was as active as the combination of RMP-PZA administered daily for three months or INH administered alone daily for six months. These results suggested that RPT-INH administered once weekly for six months might represent a fully supervisable alternative to INH self-administered daily for six months (8).
In striking contrast to the results in normal mice, almost all of the nude mice relapsed and died during the first three months after stopping treatment, regardless of the regimen that had been employed, suggesting that, in the severely immunodeficient host, tuberculosis chemoprophylaxis must be life-long. As long as the treatment was continued, once-weekly RPT alone or RPT-INH displayed significant bactericidal activity against M. tuberculosis, and RPTINH administered once every 2 weeks prevented an increase of the bacterial population. Thus, these three regimens may be considered for life-long preventive therapy in HIV-positive individuals (8).
The results of more recent experiments (11,20) also demonstrate that multidrug regimens that include once-weekly RPT-INH are highly effective during the initial and continuation phases of treatment of active tuberculosis, significantly facilitating implementation of the strategy of "directly observed treatment, short-course" (DOTS).
Because of the very promising results of experimental regimens that included once-weekly RPT, either alone or in combination with INH (8,20,28), their possible contribution to tuberculosis chemoprophylaxis is currently under investigation. An international trial of RBT chemoprophylaxis in HIV-infected patients was terminated prematurely when the sponsoring company decided that further development of the drug was financially unattractive (19).
M. AVIUM COMPLEX INFECTION
Disseminated MAC infection is the third most common opportunistic infection affecting patients with AIDS in the United States. In a nationwide survey, it was found to have occurred in 22 percent of AIDS patients (33). The incidence increases linearly over time, at a rate of 20 to 25 percent per year, after the patient's first AIDS-defining event, and increases exponentially as the CD4 cell count approaches zero (40). Evidence suggests that MAC may eventually infect most if not all HIV-infected patients who do not die from other HIV-related events (40). Because MAC infection contributes substantially to the morbidity and mortality of AIDS patients (23,40), chemoprophylaxis against MAC infection appears to be mandatory. Unfortunately, MAC is susceptible to no more than a handful of antimicrobial agents To date, only RBT, clarithromycin (CLA), and azithromycin (AZI) have been shown to be effective in preventing MAC infection.
MAC infection of the beige mouse has been widely employed for experimental work in chemotherapy. Perhaps because beige mice are highly susceptible to MAC, a technique for establishing self-limited multiplication of MAC, a basic requirement for the study of chemoprophylaxis against MAC infection in animals, remains to be developed. Consequently, practically no studies of prevention of MAC infection have been carried out in experimental animals, and all of the information collected thus far has been generated from human trials.
Rifabutin. RBT was the first drug shown to be effective in preventing MAC infection (39). In two randomized, double-blind trials, AIDS patients with CD4 cell counts no greater than 200 per mm3 were treated with either 300 mg RBT daily or placebo. In the first trial, MAC bacteremia developed in 51 (17 percent) of 298 patients in the placebo group and 24 (8 percent) of 292 patients in the RBT group (p <0.001). In the second trial, bacteremia developed in 51 (18 percent) of 282 patients in the placebo group and 24 (9 percent) of 274 patients in the RBT group (p = 0.002) (39).
Whereas these trials indicated that chemoprophylaxis with RBT was capable of reducing the frequency of disseminated MAC infection in patients with AIDS, two subsequent trials demonstrated that RBT was less effective than either AZI (21) or CLA (3) in preventing MAC infection. One of the features of treatment or prophylaxis with RBT is that drug resistance has never been observed (21,23,39), although we know that spontaneously occurring RBT-resistant mutants exist among the MAC population (unpublished data). This phenomenon suggests that the bactericidal activity of RBT is so modest, that it is unable to select the resistant mutants during treatment with RBT alone.
Clarithromycin. In one study (7), more than 50 percent of patients treated with CLA experienced gastrointestinal (GI) side effects, which were dose-related. Because of side effects, almost 40 percent of AIDS patients treated with 2000 mg CLA twice daily and more than 10 percent of those treated with 1000 mg CLA twice daily had to discontinue treatment prematurely; consequently the survival rate was significantly longer among patients treated with 500 mg CLA twice daily than among those administered CLA in the larger dosages. Therefore, no more than CLA 500 mg twice daily should be administered for chemoprophylaxis of MAC infection (23).
In a double-blind, placebo-controlled trial, 667 patients with advanced AIDS and a CD4 count no greater than 100 per ram' were randomly allocated to one of two groups, and administered 500 mg CLA or an identical-appearing placebo twice daily for an average of 10.5 and 9.5 months, respectively (46)- MAC infection, confirmed by blood culture, developed in 19 (6 percent) of the 333 patients in the CLA group and 53 (16 percent) of the 334 patients in the placebo group; thus, administered in this dosage, CLA prevented two-thirds of the expected cases of MAC infection. During a follow-up period of about ten months, 32 percent of the patients in the CLA group and 41 percent of those in the placebo group died (hazard ratio, 0.75; p = 0.026).
The protective effect of CLA was also demonstrated in a large prospective trial in which the effects of CLA alone, RBT alone and CLA-RBT were compared (3). CLARBT was not shown to be more effective than CLA alone; the likely explanation for the lack of additional benefit of the combination was a drug-drug interaction between CLA and RBT that reduced serum levels of CLA (23).
As was demonstrated in curative studies in humans (7) and in beige mice (29), acquired CLA-resistance was detected in 29 to 58 percent of patients administered CLA as prophylaxis (23,46).
Azithromycin. Azithromycin (AZI) has an extraordinarily long half-life-57 hours in man (l2)-which permits weekly administration of the drug (21,44), although some workers believe that the optimal dosage schedule of AZI remains to be determined (23).
In a trial among 693 AIDS patients with CD4 no greater than 100 per mm', three prophylactic regimens were compared (21). Patients were administered either 300 mg RBT daily, 1200 mg AZI once weekly, or both drugs. After one year, the incidence of disseminated MAC infection was 15.3 percent with RBT, 7.6 percent with AZI, and 2.8 percent with the combination. The risk of disease among those administered AZI was half that among those administered RBT (hazard ratio, 0.53; p = 0.008), and was even lower when the two-drug regimen was compared with RBT alone (hazard ratio, 0.28; p <0.001) or AZI alone (hazard ratio, 0.53; p = 0.03).
AZI in a dosage of 1200 mg once weekly was compared with placebo in another double-blind trial (44). In an intent-totreat analysis through the end of therapy plus 30 days, 9 (10.6 percent) of the 85 patients in the AZI group and 22 (24.7 percent) of the 89 patients in the placebo group developed MAC infection (hazard ratio, 0.34; p = 0.004).
The tolerability of treatment by the three drugs was surprisingly similar: 8 to 9 percent of patients were forced to discontinue treatment prematurely because of GI side effects, the toxic effect that most frequently limited dosage (21,44,46). Even when AZI was administered once weekly, 78.9 percent of patients experienced GI side effects (44). Combining AZI with RBT increased the frequency of dose-limiting toxicity by two-thirds (21).
Administering these drugs together with other medications used to treat patients with advanced AIDS has substantial pharmacokinetic implications. Serum levels of both CLA and RBT increase when these are administered together with fluconazole; HIVprotease inhibitors increase the metabolism of CLA and decrease that of RBT (21,23). The clinical importance of these interactions has not been determined, but a patient taking indinavir should reduce the dose of RBT by half, whereas a patient taking ritonavir should avoid RBT altogether (23). AZI has not been reported to interact with protease inhibitors, but it has been less well studied.
With respect to the cost of the available regimens, once-weekly AZI is least expensive, daily CLA or RBT is more expensive, and the combination AZI-RBT is most expensive.
In summary, the choice of antimicrobial agents for chemoprophylaxis of MAC infection is very limited. Although the U.S. Public Health Service recommended either CLA or AZI as the preferred prophylactic agent for MAC infection (34), neither of these drugs is highly effective or well tolerated. Although combined therapy may be more effective than monotherapy for reducing the risk of MAC infection, and may have a role in patients with very low CD4 counts (<10 per mm3), disadvantages of the combined treatment include more frequent instances of intolerance, higher cost, and greater potential of drug-drug interaction (21). Clearly, new antimicrobial agents are needed to prevent or treat MAC infection.
LEPROSY
The epidemiology of leprosy indicates that to prevent a single case of leprosy hundreds or thousands must be treated. It is unlikely, therefore, that chemoprophylaxis will become a routine method of leprosy control. However, chemoprophylaxis may play a role in special situations, such as in isolated, hyperendemic "pockets." With very few exceptions, the health infrastructure and MDT services in these pockets are weak. Therefore, unless the regimen is very simple, it will be virtually impossible to apply to chemoprophylaxis of leprosy.
Sulfone chemoprophylaxis
After dapsone became available for the treatment of leprosy, a number of trials were conducted in several endemic areas, particularly in Asia, to evaluate the possibility of using dapsone for prevention of leprosy (41-43). Although the dosages and duration of treatment varied widely, in general, the drug was administered in a dosage of 50 mg once or twice weekly for children aged 11-15 years. Later, acedapsone was also tested, but on a smaller scale; the dosage of acedapsone was 225 mg intramuscularly every 10-11 weeks for children 6-15 years of age (43, 47-49). Because both dapsone and acedapsone display only weak bactericidal activity against M. leprae (53), the duration of prophylactic treatment was more than three years, which caused operational difficulties. The results of the trials indicated that chemoprophylaxis with a sulfone had a protective efficacy of about 50 percent (43). It was unclear whether sulfone chemoprophylaxis was capable of preventing the occurrence of MB leprosy, and the long-term effects remain to be determined.
Because of the operational difficulties and the modest efficacy of sulfone prophylaxis, the chemoprophylaxis of leprosy claimed very little interest after the introduction of MDT. It is only recently, primarily in the Western Pacific region, that there has been renewed interest in leprosy chemoprophylaxis.
Newer prophylactic regimens against leprosy
Today, chemoprophylaxis with a sulfone is no longer appropriate. Its bactericidal activity is too weak, requiring a long duration of treatment, one of the main sources of operational difficulties, particularly poor compliance. Second, dapsone-resistant M. leprae are now ubiquitous.
To develop newer prophylactic regimens, we began from the following working assumptions: a) by definition, a person with subclinical leprosy infection is skin-smear negative and, therefore, harbors no more than 106 M. leprae in his body; b) because, in previously untreated lepromatous patients, the great majority of organisms are dead (18,32), one may assume that no more than 10 percent of the organisms in the subclinically infected subject are viable- i.e., the total number of viable organisms in the body is no greater than 105; c) in the bacterial population of a previously untreated lepromatous patient, the frequency of spontaneously occurring RMP-resistant mutants is no greater than 1:107 (25) and, therefore, it is very unlikely that the bacterial population of a subclinically infected subject includes a single RMP-resistant mutant; and d) based upon studies among contacts of leprosy patients, at least 90 percent, and probably more than 95 percent, of subclinical leprosy infections subside spontaneously but, on the other hand, some such infections give rise to overt MB cases. Based on these assumptions, we propose two principles for the newer regimens: a) the drug(s) should be administered in no more than a single dose and b) the regimen should always contain RMP (see below).
For chemoprophylaxis of leprosy, the target population is composed of healthy and asymptomatic, subclinically infected subjects; they do not need and would not accept treatment as leprosy patients. Furthermore, the financial impact and other operational considerations also prohibit treatment of these subjects by regimens similar to those employed for patients.
Recently, a double-blind trial among patients with single-lesion paucibacillary (PB) leprosy demonstrated that, as measured by clinical improvement, a single dose of the combination 600 mg RMP, 400 mg ofloxacin (OFLO), and 100 mg minocycline (MINO) (ROM) was almost as effective as six months of the standard MDT regimen (54). These results led the WHO Expert Committee on Leprosy, at its most recent meeting, to recommend a single dose of ROM as an acceptable and cost-effective alternative regimen for the treatment of patients with single-lesion PB leprosy (59). Because it is very unlikely that the size of the bacterial population in the great majority of subclinically infected subjects is greater than that in patients with single-lesion PB leprosy, the results of the trial and this recommendation by the WHO Expert Committee suggested that a single-dose approach might also be applied to the chemoprophylaxis of leprosy, greatly reducing the operational difficulties posed by long-duration sulfone chemoprophylaxis.
At present, four drugs-RMP, OFLO, CLA and MINO-may be considered as candidates for inclusion in the newer regimen(s). Numerous experiments (l6,26,27,30) and clinical trials (9,14,31,37,51,52) have convincingly demonstrated that RMP exerts a very rapid and powerful bactericidal action against M. leprae; a single dose of 10 mg RMP per kg body weight kills 90-99 percent of M. leprae in mice and humans, and its activity is significantly greater than that of any combination of other drugs (30,31). Because RMP is by far the most bactericidal drug against M. leprae, and is very well tolerated when it is administered once monthly, the newer regimen(s) must include RMP.
Administration to mice of a single dose of 100 mg CLA per kg or 50 mg MINO per kg (60), or to patients of 800 mg OFLO (18) or 200 mg MINO (15) showed measurable but modest bactericidal effects (32). Single doses of various combinations of the three drugs, e.g., CLAMINO, with or without OFLO, in mice (30) and in patients (3l) were more effective than monotherapy with any of the components alone, and similar in effectiveness to a month's administration of daily dapsone plus clofazimine; however, GI side effects occurred in 85 percent of the patients, most likely caused by the larger dosage of CLA in the combinations. Therefore, chemoprophylaxis regimens should not include CLA.
The activity of a single dose of OFLOMINO (OM) was dose-related in mice; the smaller dosage had no bactericidal effect, whereas the larger dosage-300 mg OFLO plus 50 mg MINO per kg-killed 84-90 percent of the M. leprae in mice (32). A single dose of 400 mg OFLO plus 100 mg MINO displayed bactericidal effects in seven of 10 lepromatous patients, with median killing of 76 percent of the organisms. The single dose of OM was well tolerated; the GI side effects were relatively few and mild (32).
Because of its moderate activity and good tolerability, OFLO-MINO was combined with RMP for testing in mouse experiment and clinical trial. A single dose of ROM killed 96.8-98.0 percent of M. leprae in mice, and at least 95.7 percent of the organisms in nine of 10 previously untreated lepromatous patients (32), indicating that a single dose of ROM is highly bactericidal against M. leprae in mice and humans. However, ROM was no more active than RMP alone (32).
Despite the modest bactericidal effect of a single dose of OM, the administration of multiple doses might play a crucial role in eliminating the RMP-resistant mutants, of which there are estimated to be no more than 104 in the bacterial population of a previously untreated lepromatous patient (25). Therefore, ROM may be employed as a fully supervisable, monthly administered regimen for the treatment of leprosy (32); its efficacy for both MB and PB leprosy and its tolerability are being evaluated in several field trials in leprosy-endemic countries.
A convenient regimen for leprosy chemoprophylaxis is a single dose of ROM, as is used for the treatment of single-lesion PB leprosy (54,59). However, a single dose of ROM appears to be no more effective than a single dose of RMP alone. And the additional drugs are not required to prevent the emergence of RMP-resistant mutant M. leprae. Moreover, the addition of OM to RMP will increase the cost of chemoprophylaxis and the risk of side effects. Therefore, the alternative is a single dose of 600 mg RMP alone. Whether or not either regimen is capable of preventing the occurrence of overt leprosy may be answered only by a controlled field trial.
In the only published trial of RMP chemoprophylaxis of leprosy, a single dose of RMP alone was tested in the Southern Marquesas Islands, a hyperendemic area with an annual detection rate of 48.9 per 100,000 inhabitants (4,5). In 1988, in addition to the provision of MDT for all known cases of leprosy, 2751 (98.7 percent) of the 2786 inhabitants of the Southern Marquesas and 3144 people in the Northern Marquesas, who were either born in the Southern Marquesas or whose family originated there, were administered RMP in a dosage of 25 mg per kg. "Cutaneous reactions," consisting of transitory flushing and itching, occurred among 2.9 percent of those treated (4). During four years of follow up, two leprosy patients-one smear-positive borderline (BB-BL) and the other smear-negative tuberculoid-were detected 4 and 21 months, respectively, after the dose of RMP, but only one of them-the smearnegative-was considered a failure of chemoprophylaxis (5). Because his borderline lesion was located at the site of a discolored patch that had appeared 9 months before and disappeared 6 months before chemoprophylaxis, the smear-positive patient was considered a missed case (4-5). Considering the diminishing detection rate among the entire population in French Polynesia, it was estimated that the effectiveness of chemoprophylaxis with a single dose of 25 mg RMP per kg was about 40-50 percent (5). Because the population was small and the duration of follow-up short, one cannot come to a conclusion with respect to its protective efficacy (5).
Almost 10 million leprosy patients have been treated with MDT including 600 mg (approximately 10 mg per kg) RMP once monthly, which has been well tolerated. On the other hand, larger doses of RMP, such as 1500 mg (approximately 25 mg per kg), had been tested in only a few clinical trials in which the drug was often combined with daily dapsone (6,14,17,24,50). Similar to that observed in patients treated with a single 600-mg dose of RMP, a profound bactericidal effect was observed virtually immediately after a single 1500-mg dose of RMP (14). However, the effectiveness of the two different dosages has never been directly compared in a clinical trial. Until there is clear evidence that a single 1500-mg dose of RMP is more bactericidal than, and as well tolerated as, a 600-mg dose, RMP should be administered for chemoprophylaxis in a dose of 600 mg.
A major concern with respect to the chemoprophylaxis of leprosy is its efficacy in preventing the occurrence of MB leprosy. In the trials of sulfone chemoprophylaxis, not enough MB patients occurred among the untreated control group to permit measurement of the efficacy of the chemoprophylaxis (43), nor is there information about the efficacy of a single dose of RMP in preventing or reducing the occurrence of such cases. In two clinical trials (6,24), which were designed to measure the rate of relapse, skin-smear-negative MB patients who had been treated for long periods by dapsone monotherapy were administered a single 1500-mg dose of RMP immediately before stopping all chemotherapy. The results indicated that a single dose of RMP neither prevented relapse nor reduced its frequency among MB patients who had already become clinically and skin-smear negative after dapsone monotherapy. Of course, the population of viable M. leprae in skin-smear negative lepromatous patients may be different from that of subclinically infected subjects who tend to evolve toward MB leprosy; therefore, the efficacy of a single dose of RMP in preventing relapse among the smear-negative patients may be different from that in preventing the development of overt MB leprosy in the subclinically infected. Whether chemoprophylaxis with RMP can prevent the development of MB leprosy can be determined only by field trials.
Finally, concern has been expressed with respect to the potential risk of emergence of RMP-resistant M. leprae by chemoprophylaxis with a single dose of RMP. As mentioned earlier, the bacterial population of a subclinically infected person is small, and is unlikely to include a single RMP-resistant mutant; therefore, the risk of RMP resistance is negligible. On the other hand, if, for whatever reason, the bacterial population should be larger than expected, and even if it includes RMP-resistant mutants, the emergence of RMP-resistance is still very unlikely because a single dose of RMP is insufficient to select the resistant mutants, as has been shown in MB patients who relapsed after a single dose of RMP (17,24,38).
DISCUSSION
Prof. Levy: In the trial of chemoprophylaxis in the Southern Marquesas, RMP was administered in a dosage of 25 mg per kg. Is there any evidence that the drug administered in this dosage is more active than in a dosage of 10 mg per kg?
Prof. Ji: As I remember, only two papers have appeared that deal with RMP in a dosage of 25 mg per kg-one by Levy and Shepard, and the second by Gelber, et al. Certainly, RMP is very active in this dosage, but there is no evidence to suggest that it is more active in this dosage than in a dosage of 10 mg per kg. I'm very concerned about potential side effects. We know from the experience with millions of doses of MDT that RMP is well tolerated when it is administered in a dosage of 10 mg per kg, which is equivalent to 600 mg in man. On the other hand, there has been only limited experience with RMP administered in a dose of 1500 mg, the equivalent in man of a dosage of 25 mg per kg.
Dr. Takashima: How efficacious is RMP against MAC?
Prof. Ji: The results have varied from laboratory to laboratory. In our laboratory, none of the rifamycins has been found to be clearly active against MAC. Moreover, that no patient administered rifabutin monotherapy relapsed with the emergence of drug-resistant organisms is most unusual, especially in view of the presence of spontaneous drug-resistant mutants in a frequency of about 10-8. This suggests that the rifamycins are only weakly bactericidal against MAC.
Dr. Izumi: Our work with the serodiagnosis of subclinical M. leprae infection in Indonesia suggests that the number of organisms harbored by a subclinically infected individual may be much larger than the figure you gave. In Norway, I believe, it was not possible to produce anti-PGL-I antibodies in human volunteers with 107 killed M. leprae, and 10s organisms were required. We have demonstrated that 30 percent of the population of an endemic area in Indonesia carry these antibodies and are, we believe, subclinically infected.
Prof. Ji: I think that we are not yet able to correlate the number of M. leprae in the bacterial population with the degree of seropositivity to PGL-I. Second, keep in mind that, in the event that the patient with PB leprosy was originally infected with dapsone-resistant organisms, something that occurs in approximately half of MB patients, MDT (monthly RMP plus daily dapsone) is really monotherapy with RMP. If the bacterial population were much larger than 106, we should expect relapse with the emergence of RMP-resistant organisms. Not one such relapse has been reported among the millions of PB patients who have been administered MDT.
REFERENCES
1. AMERICAN THORACIC SOCIETY and CENTERS FOR DISEASE CONTROL and PREVENTION. Treatment of tuberculosis and tuberculosis infection in adults and children. Am. J. Respir. Crit. Care Med. 149 (1994) 1359-1374.
2. ANONYMOUS. Chemoprophylaxis for tuberculosis. Tubercle 61 (1981)69-72.
3. BENSON, C.A., COHN, D.L., WILLIAMS, P. and ACTG 196/CPCRA 009 STUDY TEAM. A phase III prospective, randomized, double-blind study of the safety and efficacy of clarithromycin vs. rifabutin vs. clarithromycin plus rifabutin for prevention of Mycobacterium avium complex disease in HIV + patients with CD 4 count less than or equal to 10 0 cell/ul [abstract no. 205] . in: Program and Abstracts of the 3rd Conference on Retroviruses and Opportunistic Infections (Washington, D.C.) . Alexandria, Virginia: Infectious Diseases Society, 1996.
4. CARTEL, J.L., CHANTEAU, S., BOUTIN, J.P., TAYLOR, R., PLICHART R., ROUX, J., CELERIER, P. AND GROSSET, J.H. Implementation of chemoprophylaxis of leprosy in the Southern Marquesas with a single dose of 25 rag per kg rifampin. int. J. Lepr. 57 (1989) 810-816.
5. CARTEL, J.L., CHANTEAU, S., MOULIA-PLLAT, J.P., PLICHART, R., GLAZIOU, P., BOUTIN, J.P., ROUX, J. AND GROSSET, J.H. Chemoprophylaxis of leprosy with a single dose of 25 mg per nig rifampin in the southern Marquesas; results after four years. Int. J. Lepr. 60(1992)416-420.
6. CARTEL, J.L. and NAUDIN, J.C. Rate of relapse in multibacillary patients after cessation of longcourse dapsone monotherapy supplemented by a final supervised single dose of 1500 mg of rifampin. Int. J. Lepr. 62 (1994) 215-219.
7. CHAISSON, R.E., BENSON, C.A., DUBE, M.P., HEIFETS, L.B., KORVICK, J.A., ELKIN, S., SMITH, T, CRAKT, J.C, SATTLER, F.R. and the AIDS CLINICAL TRIALS GROUP PROTOCOL 157 STUDY TEAM. Clarithromycin therapy for bacteremic Mycobacterium avium complex disease. ann. intern. med. 121 (1994) 905-911.
8. CHAPUIS, L., JI, B., TRUEI OT-PERNOT, C, O'BRIEN, R.J., RAVIGLIONE, M. C and GROSSET, J.H. Preventive therapy of tuberculosis with rifapentine in immunocompetent and nude mice. am. J. Respir. Crit. Care Med. 150 (1994) 1355-1362.
9. COLLABORATIVE EFFORT OF THE U.S. LEPROSY PANEL (U.S.-JAPAN COOPERATIVE MEDICAL SCIENCE PROGRAM) and THE LEONARD WOOD MEMORIAL. Rifampin therapy of lepromatous leprosy. Am. J. Trop. Med. Hyg. 24 (1975) 475-484.
10. COMSTOCK, G.W. and WOOLPERT, S.F. Preventive therapy. In: kubica, g.p. and wayne, l.g.,eds. the mycobacteria. a sourcebook. new york: marcel dckker, inc., (1994, PP. 1071-1082.
11. DANIEL, N., LOUNIS, N., JI, B.. O'BRIEN, R.J., VERNON, A., GEITER, L.J., SZPYTMA, M., TRUFFOTPERNOT, C, HEJBLUM, G. and GROSSET, J. Further studies on once-weekly rifapentine-containing regimens for treatment of tuberculosis in mice (submitted for publication).
12. DUNN, C. J. and BARRADELL, LB . Azithromycin. a review of its pharmacological properties and use as 3-day therapy in respiratory tract infection. Drug 51 (1996)483-505.
13. FEREBEE, S. H. Controlled chemoprophylaxis trials in tuberculosis. A General review. Adv. Tuberc. Res. 17(1970) 29-106.
14. GELBER, R.H. AND LEVY, L. Detection of persisting Mycobacterium leprae by inoculation of the neonatally thymectomized rat. Int. J. Lepr. 55(1987) 872-878.
15. GELBER, R.H., MURRAY, L.P., SIU, P., TSANG, M. and REA, T.H. Efficacy of minocycline in single dose and at 10 0 mg twice daily for lepromatous leprosy. Int. J. Lepr. 62 (1994) 568-573.
16. GROSSET, J.H. AND GUEI.PA-LAURA.S, C.C. Activity of rifampin in infection of normal mice with Mycobacterium leprae. Int. J. Lepr. 55 (1987) 847-851.
17. GROSSET, J.H., GUELPA-LAURAS, C.C , BOBIN, P., BRLCKER, G., CARTEL, J.L., CONSTANT-DESPORTES, M., FLAGEUL, B., FREDERIC, M., GUILLAUME, J.C. and MILIAN, J. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin. Int. J. Lepr. 57 (1989) 607-614.
18. GROSSET. J.H., JI, B., GUELPA-LAURAS, C.C , PERANI, E.G. AND N'DLLI, L. Clinical trials of pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58 (1990) 281-295.
19. GROSSET, J.H. AND O'BRIEN, R.J. Advances in tuberculosis preventive therapy. Seminars Respir. Crit. Care Med. 18 (1997) 449-457.
20. GROSSET, J., LOUNIS, N., TRUFFOT-PERNOT, C, O'BRIEN, R.J., RAVIGLIONE, M.C. and JL, B. Onceweekly rifapentine-containing regimens for treatment of tuberculosis in mice. Am. J. Respir. Crit. Care Med. 157 (1998) 1436-1440.
21. HAVLIR, D.V., DUBE, MP. , SATTLER, F.R., FORTHAL, D.N., KEMPER, CA., DUNNE, M.W., PARENTI, D.M., LAVELLE, J.P., WHITE JR., A.C., WHIT, M.D., BOZZ.ETTE, S.A. AND MCCUTCHAN, J.A. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. N. Engl. J. Med. 335 (1996) 392-398.
22. HONG KONG CHEST SERVICE/TUBERCUI OSIS RESEARCH CENTER, MADRAS/BRITISH MEDICAL RESEARCH COUNCIL. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am. Rev. Respir. Dis. 145 (1992) 36-41.
23. HORSBURGH, CR., JR. Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. New Engl. J. Med. 324 (1991) 1332-1338.
24. JAMET, P., BLANC, L., FAYNE, O.C, TRAORE, I. AND BOBIN, P. Relapses after a single dose of rifampin in skin-smear negative multibacillary patients after dapsone monotherapy. Int. J. Lepr. 62 (1994) 209-214.
25. JI, B. and GROSSET, J. Recent advances in the chemotherapy of leprosy. Lepr. Rev. 61 (1990) 313-329.
26. JI, B., PERANI, E.G. and GROSSET, J.H. Effectiveness of clarithromycin and minocycline alone and in combination against experimental Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35 (1991) 579-581.
26. Ji, B., PERANI, E.G.. PEETINON, C. and GROSSET, JH. Bactericidal activities of single or ntullipledoses of various contbinations of new antileprosydrugs and/or rifampin against M. leprae in atice.Int. J. Lepr. 60 (1992) 556-561.
28. JI, B., TRUFFOT-PERNOT, C., LACROIX, C., RAVIGLIONE, M.C., O'BRIEN, R.J., OLLIARO, P.,ROSCIGNO, G. and GROSSET, J. Effectiveness of ri-fampin, rifabutin, and rifapentine for preventivethcrapy of tuberculosis in coice. Ana. Rev. Respir.Dis. 148 (1993) 1541-1546.
29. Ji, B., LoENis, N., TRUttoT-PvRNOT, C. and GRos-sEr, J.H. Effectiveness of various antimicrobialagents against M eabacierium aviam contplex inthe beige ntouse mode!. Antinticrob. AgentsChemother. 38 (1994) 2521-2529.
30. Ji, B., PERANI, E.G., PETINON, C. and GROSSET, JH. Bactericidal activities of conabinations of newdrugs against Mycobacterirun leprae in nade atice.Antimicrob. Agents Chemother. 40 (1996)393-399.
31. Ji, B., JAMET, P., PERANI, E.G., Sow, S., LIEN-HARDT , C., PETINON, C. and GROSSET, J.H. Bactericidal activity of a single dose of clarithronaycinplus minocyclinc, with or without ofloxacin,against Mvcobacteriran leprae in patients. Antimicrob. Agents Chemother. 40 (1996) 2137-2141.
32. Ji, B., Sow, S., FERAS', E., LIENHARDT, C.,DiDEROT, V. and GRossEr, J. Bactericida! activityof a single dose contbinatioa of otloxacin plusminocyclinc, with or without rifampin, against Mycobacteriunr leprcre in atice and ia leprontatouspatients. Antinticrob. Agents Chemother. 42(1998)1115-1120.
33. JONES, J.I., HANSON, D.L., CHU, S.Y., FLENIING,P.L., Hu, D.J. and W,\RD, J.W. Surveillance of AIDS-defining conditions ia the United States.AIDS 8 (1994) 1489-1493.
34. KAPLAN, J.E., MASUR, H. and HOLMES, K.K.USPHS/IDSA guidelines for the prevention of op-portunistic infections in persons infected with human immunodeficiency virus. Morb. Mortal.Wkly. Rep. 46 (1997) 1-46.
35. KOPANOFF, D.E., SNIDER, D.E. and CARAS, G.J.Isoniazid-related hepatitis. A US Puhlic Health Service cooperative surveillance study. Ant. Rev.Respir. Dis. 117 (1978) 991-1001.
36. LECOEUR. H.F., TRUFFOT-PERNOT, C. and GROSSET,J.H. Experimental short-course preventive therapyof tuberculosis with rifampin and pyrazinantide.Ant. Rev. Respir. Dis. 140 (1989) 1189-1193.
37. LEVY, L., SHEPARD, C.C. and FANAL, P. The bactericidal effect of rifampin on M. leprae in man: (a) single doses of 600, 900 and 1200 ntg; and (h)daily doses of 300 mg. Int. J. Lepr. 44 (1976) 183-187.
38. MARCUOUX CHEMOTHERAPY STUDY GROUP. Relapses in multibaccilary leprosy patients after stop-ping treatment with rifampin-containing combined regimens. Int. J. Lepr. 60 (1992) 525-535.
39. NIGHTINGALE, S.D.. CAMERON, D.W., GORDIN, F.M., SULI.AM, P.M., COHN, D.L., CHAISSON, R.E., ERON, L.J., SI'ARII, P.D., BIHARI, B. AND KAUFMAN, D.L . TWO CONTROLLED TRIALS OF RIFABUTIN PROPHYLAXIS AGAINST Mycobacterium avium COMPLEX INFECTION IN AIDS. N. ENGL. J. MED. 329 (1993) 828-833.
40. NIGHTINGALE, S.D. PROPHYLAXIS AGAINST Mycobacterium avium-iiitracellulare COMPLEX INFECTIONS IN HUMAN IMMUNODEFICIENCY VIRUS-INFECTED PATIENTS. INFECTION 25 (1997) 67-70.
41. NOORDEEN, S.K. Chemoprophylaxis in leprosy. Lepr. India 40 (1968) 115-119.
42. NOORDEEN, S. K. Long-term effects of chemoprophylaxis among contacts of lepromatous cases. Results of a 8 1/2 years follow-up. Lepr. India 49 (1977)504-509.
43. NOORDEEN, S. K. Chemoprophylaxis. Health Coop. Papers 1 (1982) 173-182.
44. OLDI IELD, E.C., III, FESSEL, W.J., DUNNE, M.W., DICKINSON, G., WALLACE, M.R., BYRNE, W., CHUNG, R., WAGNER, K.F., PAPARELLO, S.F., CRAIG, D.B., MEI.CHER, G., ZAJDOWICZ, M., WILLIAMS, R.F., KELLY, J.W., ZELASKY, M., HEIIETS, L.B. and BERMAN, J.D. Once weekly azithromycin therapy for prevention of Mycobacterium avium complex infection in patients with AIDS. A randomized, double-blind, placebo-controlled multicenter trial. Clin. Infect Dis. 26 (1998)611-619.
45. PALMER, C.E., FEREBEE, S.H., and HOPWOOD, L. Studies on prevention of experimental tuberculosis with isoniazid. II. Effects of different dosage regimens. Am. Rev. Tuberc. 74 (1956) 917-939.
46. PIERCE, M., CRAMPTON, S., HENRY, D., HEIIETS, L., LAMARCA, A., MONTECALVO, M., WORMSER, G.P., JABLONOWSKI, H., JEMSEK, J., CYNAMON, M., YANGCO, B.G., NOTARIO, G., and CRAFT, J.C. A randomized trial of clarithromycin as prophylaxis against disseminated Mycobacterium avium complex infection in patients with advanced acquired immunodeficiency syndrome. N. Engl. J. Med. 335(1996)384-391.
47. RUSSELL, D.A., SIIEPARD, C.C, MCRAE, D.H., SCOTT, G.C. and VINCIN, D.R. Acedapsone (DADDS) treatment of leprosy patients in the Karimui of Papua-New Guinea: status at six years. Am. J. Trop. Med. Hyg. 24 (1975) 485-495.
48. RUSSELL, D.A., WORTH, R.M., SCOTT, G.C, VINCIN, D.R., JANO, B., FASAL, P. and SIIEPARD C.C. Experience with acedapsone (DADDS) in the therapeutic trial in New Guinea and the chemoprophylactic trial in Micronesia. Int. J. Lepr. 44(1976) 170-176.
49. RUSSELL, D.A., WORTH, R.M., JANO, B., FASAL, P. and SIIEPARD, C.C. Acedapsone in the prevention of leprosy; field trial in three high prevalence villages in Micronesia. Am. J. Trop. Med. Hyg. 28 (1979)559-563.
50. SUBCOMMITTEE ON CLINICAL TRIALS OF THE CHEMOTHERAPY OF LEPROSY (THELEP) SCIENTIHC WORKING GROUP OF THE UNDP/WORLD BANK/WH O SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASE. Persisting Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58 : (1987)325-337.
51. SHEPARD, C.C., LEVY L. and FASAL, P. 1972. Rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 21 (1972) 446-449.
52. SMEPARD, C.C., LEVY L. and FASAL, P. Further experience with the rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23(1974) 1120-1124.
53. SHEPARD, C.C . A brief review of experience with short-term clinical trials monitored by mousefoot-pad inoculation. Lepr. Rev. 52 (1981) 299-308.
54. SINGLE-LESION MULTICENTRE TRIAL GROUP. Efficacy of single dose multidrug therapy for the treatment of single-lesion paucibacillary leprosy. Indian J. Lepr. 69 (1997)121-129.
55. SNIDER, D.E.JR., CARAS, G.L., and KOPLAN, J.P. Preventive therapy with isoniazid. J. Am. Med. Assoc. 255 (1986) 1579-1583.
56. SNIDER, D.E., JR. and CARAS, G.J. Isoniazid-associated hepatitis deaths: a review of available information. Am. Rev. Respir. Dis. 145 (1992) 494-497.
57. VlLLARINO, M.E., RID/ON, R., WEISMULLER, PC, EECOCK, M., MAXWELL, R.M., MEADOR, J., SMITH, P.J., CARSON, M.L. and GETTER, L.J. Rifampin preventive therapy for tuberculosis infection. Experience with 157 adolescents. Am. J. Respir. Crit. Care Med. 155 (1997) 1735-1738.
58. WIIAI.EN, C.C, JOHNSON, J.L., OKWERA, A., HOM, D.L., HUEBNER, R., MUGYENYI, P., MUGERWA, R.D. and ELLNER, J.J. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. N. Engl. J.Med. 337(1997)801-808 .
59. WORLD HEALTH ORGANIZATION. WHO Expert Committee on Leprosy. Seventh Report. WHO Technical Report Series no. 874 . World Health Organization, Geneva, (1998) .
60. XIONG, J.H., Ji, B., PERANI, E.G., PETINON, C. and GROSSET, J.H. Further study of the effectiveness of single doses of clarithromycin and minocycline against Mycobacterium leprae in mice. Int. J. Lepr. 62(1994)37-42.
THE FUTURE OF LEPROSY ELIMINATION
S. K. Noordeen
Adyar, Chennai, South India
The topic of the future of leprosy elimination has been receiving increasing attention in recent years, largely because of great progress toward the elimination of leprosy, the result of widespread implementation of multiple drug therapy (MDT). In the pre-MDT era, leprosy was considered a perennial problem, and the approach was mostly one of containment of the disease and care of the patient, with no serious thought about the future of leprosy control. The MDT era has completely changed the situation, and today we are as much concerned about the future as we are aware of the need for maintaining the current rate of progress.
Based on current information, the World Health Organization (WHO) expects that all but ten countries will achieve the goal of elimination of leprosy at the national level by the end of the year 2000. Except for those experiencing civil strife, the remaining countries are expected to reach the goal no later than the year 2005. However, it is likely that, within these countries, leprosy-endemic pockets may remain, considering the uneven distribution of leprosy. In fact, leprosy may survive in a small way for many more years by retreating to its strongholds before completely disappearing.
In considering the future of leprosy elimination, one should not overlook the factors that contributed to the current rate of progress. These include: 1) the secular downward trends of leprosy prevalence and incidence in many parts of the world, particularly in Africa; 2) the influence of improving socioeconomic conditions in some parts of Asia; 3) the varying beneficial effects of BCG vaccination; and 4) the continuing effectiveness of dapsone monotherapy in spite of drug resistance. This is not to minimize the great contribution MDT has made toward the elimination of leprosy. In fact, MDT remains the single most effective intervention, and has completely revolutionized the leprosy scene. Apart from representing a technological revolution, MDT has also led to increased political commitment and allocation of resources, and has made possible operational simplification and improvement of leprosy control procedures. MDT has also contributed to the prevention of disability through early treatment as well as reduction of the social stigma against leprosy.
In spite of the progress made thus far, and the great potential of MDT, a significant number of countries will not be able to meet the target by the year 2000, largely because of starting from a huge prevalence base or beginning MDT services late for a variety of reasons. For these countries, the problem is not one of a failure of technology but, rather, that more time will be required to reach the goal of elimination. Countries or areas with problematic epidemiological situations are extremely few. The specific difficulties impeding progress in those countries that are not likely to reach the goal by the year 2000 are mostly operational, and are capable of being addressed, provided a commitment is made and sufficient resources allocated.
In relation to the future of leprosy elimination, what is the scenario we are likely to face? First, with diminishing numbers of patients it will be extremely difficult to sustain vertical programs or even vertical elements within general health services. With reduced vertical elements, leprosy-specific skills will have only limited availability. A consequence of this could be considerable delay in the diagnosis of leprosy, as currently happens in areas of very low endemicity. Another consequence could be diminished interest in activities directed toward the prevention of disability and rehabilitation. Progressive disappearance of leprosy is likely to lead to considerable reduction of the social stigma against leprosy, as happened with tuberculosis in Western Europe. The loss of importance of leprosy-specific vertical elements is likely to have an impact on the activities of leprosy-specific nongovernmental organizations (NGOs) and, as a consequence, their role may be diminished. Leprosy research is also likely to suffer, despite exciting developments in the basic science that could have great potential application to the study of leprosy.
The fear has been expressed that there may be a resurgence of leprosy after its incidence reaches very low levels, and after all antileprosy activities have stopped. This fear is based on the resurgence of malaria and tuberculosis. The comparison of leprosy with malaria is not valid, simply because transmission of malarial parasites is far more complex, the chain of transmission involving not only humans but also insect vectors and the environment. Until every link in the chain can be broken, it will be difficult to ensure sustained success in controlling malaria. Moreover, the pathogen of malaria is far more virulent and varied than is Mycobacterium leprae. With regard to tuberculosis, it is not entirely true that there has been a world-wide resurgence of tuberculosis; the problem is coincident infection by HIV and M. tuberculosis. The impact of this problem on the global tuberculosis picture in the world is not large, and the resurgence we see is mainly in the interest in tuberculosis control, an area very much neglected in the past. Further, the problem of tuberculosis is highly complicated by the wide spread occurrence of resistance of M. tuberculosis to most drugs, including multidrug resistance. This is fortunately not the case in leprosy, and drug resistance is unlikely to emerge as a major threat to leprosy control in the future, as long as the antimicrobial drugs are administered in combination (i.e., MDT) everywhere, and are employed primarily within public health systems.
In relation to the possible resurgence of leprosy, it has often been assumed that all antileprosy activities will come to a complete stop once the goal of leprosy elimination has been reached. This is unlikely to be the case, as, after the elimination of leprosy has been achieved, continuation of leprosy activities within the general health services is foreseen. Although this may result in some dilution of the quality of antileprosy services, it is unlikely to lead to total neglect, particularly since leprosy skills and trained personnel are not likely to disappear overnight.
A major concern is the fate of personnel trained in leprosy, whether they work for national health services, NGOs, or international organizations such as WHO. Leprosy work has always been a very comfortable niche for people devoted to the cause of leprosy, who found great satisfaction in an area neglected by others. In fact, the neglect of leprosy work by others was very convenient for the devoted, and it was not uncommon for leprosy workers to insist that leprosy patients can never be taken care of by general health workers. These devoted workers feel threatened with respect to their future, and often find it convenient to project their fears onto the future of leprosy work. I am confident that, in time, their fears will be addressed effectively, so that they won't hinder the integration of leprosy within general health services.
Finally, with respect to the main theme of this meeting- i.e., the prevention of leprosy-both immunoprophylaxis and chemoprophylaxis present interesting although very limited possibilities for the future. Certainly, even should there be considerable progress in these areas, it will not be possible to apply them on a massive scale, the only way in which they could have an impact on the leprosy situation. This is mainly because of cost-benefit considerations. With respect to a disease like leprosy, which has a relatively low attack rate, even in countries with an incidence of 1 per 1000 at risk, the cost of preventing leprosy can be disproportionately high. In this instance, it will be necessary to vaccinate or administer chemoprophylaxis to 1000 persons in order to prevent a single case of leprosy, assuming that the intervention is 100 percent effective, which is unlikely to be the case. To improve the cost-benefit ratio, it is possible to confine preventive interventions to high-risk groups confined to a geographic area or an age group. However, in terms of impact on the leprosy situation at the national level, such a limited intervention can have only a limited effect. On the other hand, there is still a place for preventive interventions in specific high-risk situations, such as that in Micronesia.
In conclusion, we should recognize that we have come a long way in our successful fight against leprosy. Although the remaining problems are important, we should be neither complacent nor despairing in dealing with them. The future of leprosy elimination is certainly bright, provided we exert the necessary efforts.
DISCUSSION
Dr. Yuasa: We in leprosy have a problem. Among ourselves, we speak about leprosy all the time, as if leprosy were the only public health problem, and as if we were able to do whatever it is that we believe needs to be done. In fact, leprosy is only one of many public health problems, and we must be careful to consider leprosy in a wider context.
NEW BIOLOGICAL TOOLS FOR LEPROSY SURVEILLANCE
Sang-Nae Cho1; Patrick J. Brennan2
1. Department of Microbiology, Yonsei University College of Medicine, Seoul, Korea.
2. Department of Microbiology, Colorado State University, Fort Collins, Colorado 80523, U.S.A.
New biological tools are required for leprosy surveillance, both for detection of Mycobacterium leprae infection and for early diagnosis of the disease, particularly in the final phases of the elimination strategy. Now that the prevalence of leprosy has declined dramatically in most leprosy-endemic countries over the last decade, incidence of the disease will become a more important measurement of true disease than prevalence for leprosy surveillance. The incidence of leprosy, however, varies markedly even within the same broad geographical region, depending on the level of effort being devoted to finding new cases, i.e., the extent of active or passive case finding. It will be difficult to adopt incidence data for leprosy surveillance throughout the leprosy-endemic world unless a uniform method of determining true incidence is employed. Therefore and alternatively, determination of the infection rate with M. leprae within a population could become an important determinant of the extent of leprosy control. In addition, much earlier diagnosis of leprosy would be of great value in preventing more severe disease, perhaps leading to disabilities, by simply initiating chemotherapy at an early stage, thereby removing the source of M. leprae transmission. New biological tools for detection of M. leprae infection and for early diagnosis of leprosy are of paramount importance in surveillance of control programs and for the ultimate eradication of the disease.
M. leprae and the biological stages of leprosy: theoretical and practical considerations. In order to develop the biological tools for detection of M. leprae infection and for early diagnosis of leprosy, more information is required with respect to both the organism and the biological stages of the disease. For example, are there important extra-human reservoirs of M. leprae, such as soil or water or nonhuman animal sources? Are strain variations important in terms of virulence, transmission rate, or the expression of major antigens in humans? For instance, the presence of M. leprae to a significant extent in extra-human reservoirs, if proven, will prove to be a major obstacle for disease eradication and will pose problems in understanding the degree of exposure to the organism related to "true" infection or transient infection and subsequent immune response in the host. It has long been claimed that the genomic DNA of M. leprae is highly conserved. However, there is now recent evidence of polymorphism. It will be important to determine whether this polymorphism is reflected in variations in virulence and transmission efficiency. Also, variations in the expression level of major antigens of M. leprae in humans, if such exist, will be reflected in the host's immune responses and will have a bearing on the extent/duration of disease and the creation of new biological tools to detect it. It remains to be explained whether variation in antibody level and T-cell responses to certain M. leprae antigens among leprosy patients is due to the difference in host response to the same antigens or due to differences in antigen expression levels between M. leprae strains, or possibly due to both. All such information related to the biology of M. leprae will help in the development of a generation of more specific and sensitive biological tools for leprosy surveillance.
Among the biological stages of leprosy, one needs more information on the infection route, degree of exposure leading to disease, incubation period, host response and clinical sequelae, and how chemotherapy can lead not only to cure, but also to relapse, lepra reactions, and re-infection. The route of M. leprae infection is an important factor affecting the host's immune responses, resulting in variations in antibody classes and levels and T-cell responses to M. leprae antigens. The degree of exposure to M. leprae has been considered as a major factor in the development of leprosy among household contacts. However, although being a household contact is a major risk factor, the majority of leprosy cases arise from situations in which there was no contact with patients. The principle of the non-symptomatic carrier in leprosy is now becoming accepted, and more information is needed, such as the infectivity of the temporary or continuous carrier. We need to know more about the meaning of the presence of M. leprae in nasal mucosa, which, thanks to the art of polymerase chain reaction (PCR) amplification, is now regarded as the most likely source of M. leprae transmission. The incubation period for leprosy varies from three to forty years, although five to ten years have been most widely cited. No information is yet available on whether or not M. leprae transmission occurs during this incubation period.
The host's immune responses to M. leprae infection and early clinical symptoms will be the most important factors in developing new biological tools for detection of infection and for early diagnosis. There is a considerable body of evidence on variations in antibody levels, subclasses, and T-cell responses to each of the known M. leprae antigens among leprosy patients with different clinical types of disease. However, more information is required for understanding the basis of such variations and the factors influencing the clinical course of the disease. Without such information, any biological tools for detection of infection and disease at the early stage will have serious limitations in terms of sensitivity and specificity.
The various chemotherapeutic regimens that have evolved over the last several decades range from dapsone monotherapy to the recently modified twelve-month multidrug therapy (MDT) for multibacillary (MB) cases and a single dose of the combination of rifampin, ofloxacin and minocycline (ROM) for patients with a single lesion. It should be noted that the majority of these regimens were evaluated based on the number of lesions rather than the bacterial index (BI). It is now important to know how the new, relatively short-course therapy regimens affect all pools of M. leprae in patients, including nasal secretions. Moreover, the existence of large numbers of bacilli, mostly dead, in tissue for the extended period of time after 12-month MDT will continuously influence immune responses. In turn, this will affect the results of the application of biological tools for monitoring the effectiveness of chemotherapy.
Relapse, lepra reactions, and re-infection after completion of MDT are issues that have attracted the attention of clinicians and research scientists in recent years, and will be important subject matters in the post-elimination period. Not much information is available about the clinical symptoms and immune responses occurring during the early stages of relapse that might be used as indicators. Only an increase of the BI, which requires viability measurements in mouse foot pads, has been consistently associated with relapse. Clinical signs of lepra reactions have been often mistakenly classified as relapse. Therefore, biological tools capable of clearly differentiating between relapse and lepra reactions will be of the utmost importance in developing new therapeutic regimens. Differentiation between relapse and re-infection has also been difficult. Proof of significant DNA polymorphism in different isolates should be invaluable in this respect. No information is yet available on the possibility of the co-existence of relapse and re-infection.
Therefore, the full understanding of the biology of M. leprae and the biological stages of leprosy are crucial requisites for a renewed effort to generate new diagnostics for the detection of M. leprae infection and for early diagnosis of the disease, particularly in the post-elimination era with a lesser disease load, more scattered endemic populations, and the relative absence of obvious, overt disease.
Pragmatics of new biological tools for leprosy . Currently available laboratory tools for leprosy surveillance include: serological assays; molecular amplification techniques; the measurement of T-cell responses, such as the skin-test response and whole blood gamma interferon (IFN-y); and molecular methods for identifying drug resistance. Among these tools, serological tests, notably those based on the M. leprae specific PGL-I antigen, have been most widely evaluated for the detection of M. leprae infection within populations as well as for early diagnosis of disease. However, due to weak antibody response at the paucibacillary [(PB), tuberculoid] end of the disease spectrum, serology is useful only in the context of multibacillary [(MB), lepromatous] leprosy, which places severe restrictions on its more widespread application. Nevertheless, a goodly portion of household contacts of MB cases have a significant level of antibodies to PGL-I and have several times greater risk of developing leprosy in the future than those with no antibodies. Thus, seropositive contacts should be major target populations for preventive therapy. Other M. leprae antigens, including M. leprae soluble antigen (MLSA), LAM, and the 36-kDa, 65-kDa, 45-kDa proteins, have also been useful in detecting M. leprae infection but, in general, show little advantage over the PGL-I antigen in terms of sensitivity and specificity. PGL-I and its neoglycoconjugates, on account of their unique specificity to M. leprae, are thus the antigens of choice in serological tests, both for measuring M. leprae infection among populations and for early diagnosis of MB cases. In addition, with the ready availability of water-soluble neoglycoconjugates containing the specific immunodeterminant of PGL-I, it is now possible to develop facile, simple "dipstick" or particle agglutination tests, particularly suitable for field use in leprosy-endemic regions.
A considerable body of data has now accumulated on the application of molecular amplification, either DNA by PCR or ribosomal RNA by NASBA, to biological questions in leprosy. Such amplification techniques have the advantage of greater sensitivity than microscopic examination, thus enabling detection of small numbers of M. leprae in clinical specimens such as nasal swabs, slit smears, and biopsy samples. These molecular techniques, however, require Highly trained personnel and expensive equipment and reagents, which only a few laboratories in the leprosy-endemic areas can afford. Even with qualified personnel and adequate facilities, it has been difficult to generate reproducible results between studies. More work is thus needed to standardize the available molecular amplification techniques.
One pragmatic use of molecular amplification is in the detection of specific mutations associated with resistance to rifampin and the fluoroquinolones. Employing these techniques, it is now possible to determine the susceptibility of isolates to these drugs in a matter of one to two days, a task that requires many months using the M. leprae infected mouse foot pad. With the pending availability of the full M. leprae genome sequence, there is the expectation that these molecular biological techniques will allow the recognition of individual strains of M. leprae, an event which should have profound consequences for epidemiological and clinical studies.
Skin tests employing MLSA [or ML-CwA (M. leprae cell wall antigens)] or other defined protein antigens are of considerable interest, because they permit one to examine T-cell rather than antibody (B-cell) responses, particularly applicable to the PB, difricult-to-diagnose aspects of the disease. MLSA, which is devoid of LAM (which suppresses the CMI response), and ML-CwA have been extensively used in in vitro assays, have passed Phase I clinical trials as skin-test antigens in humans, and are now almost ready for Phase II trials. In addition, synthetic peptides based on M. leprae-spe cific T-cell epitopes have been examined in CMI tests and show some promise. A simplified in vitro test, the so called "whole blood test" which measures IFN-y evocation in response to a variety of antigens, has been developed and evaluated in the field and shows great promise. The whole blood test has the added advantage of measuring Thl and Th2 types of responses to various antigens by detecting cytokines produced from each cell type.
In summary, over the past twenty years, there have been considerable effort and numerous approaches applied to developing biological tools for leprosy surveillance, particularly for the detection of M. leprae infection and for early diagnosis of leprosy. However, all of the ensuing developments have been marred in their more widespread application due to our limited knowledge of the biology of M. leprae and the biological stages of leprosy. For the future, we are placing much hope, perhaps too much so, in the pending completion of the M. leprae genome as a source of information on M. leprae-specific proteins, and thus potential antigens, and for evidence of genetic polymorphism. Such developments, combined with renewed interest in the "carrier state" and the immunological and bacterial basis of neuropathy and reactions, augur well for a new generation of practical tools for leprosy surveillance.
DISCUSSION
Dr. Izumi: Please explain the use of PCR in identification of strains of M. leprae.
Prof. Cho: Dr. Stewart Cole published data on two sites characterized by repetitive sequences, and we have found three more. However, fewer than 30 per cent of the total sequences of the M. leprae genome is included in the current genomic database. Hence, the availability of the entire genome sequence in the near future will reveal many more sites with repetitive sequences. The "copy numbers" of known repeats, i.e., the number of repeats in a given site, varies from 9 to 34, and these variations of copy numbers permit identification of individual strains of M. leprae.
RATIONALE FOR THE PREVENTIVE TREATMENT OF LEPROSY
M. F. Lechat
Université Catholique de Louvain, Brussels, Belgium
Multiple drug therapy (MDT) has made a major contribution to the remarkable decline of the prevalence of leprosy that has been observed in the course of the last 10 years in the context of the World Health Organization (WHO)-sponsored Leprosy Elimination Program. Now that the prevalence target of 1 case per 10,000 population has been reached in a large number of endemic countries, and others will come close to this target by the year 2000, there has arisen a tendency to consider the present MDT-based strategy as the exclusive means by which to tackle the leprosy problem. Because MDT has proven cheap, safe and effective, why then bother to explore alternative or complementary strategies, such as prevention? At times, it has been said that there is no place for prevention. MDT, together with its package of early detection, community participation and political commitment, is so successful that it could indeed make us hostages of its very success, preventing us from conducting promising research or testing innovative approaches.
There are two important reasons why prevention should not be disregarded as a component of leprosy control. One is the possibility that the MDT strategy will ultimately fail to deliver its expected results. The second is that prevention could prove more effective or less expensive than MDT as a means of controlling leprosy in special situations.
With respect to the ultimate failure of the MDT strategy, the purpose of leprosy control is to reduce and ultimately to interrupt the transmission of Mycobacterium leprae, thereby reducing the incidence to zero. Because, for a number of reasons, measurement of the incidence raises major operational difficulties, prevalence-the number of existing cases-serves as a proxy indicator of transmission. The fewer cases in a population today, the fewer the new cases in the future.
MDT is highly effective in killing M. leprae in its human reservoir, the infected individual. Therefore, it is the backbone of the ongoing world-wide elimination program, whose target, as stated in the 1991 Resolution of the World Health Assembly, is to reduce prevalence to a level below 1 case per 10,000 population. The relevance of this target to achieving the stated purpose rests on three explicit premises: 1) that the patient is the only significant source of infection; 2) that at such a low, admittedly arbitrary, level of prevalence, the potential for transmission is much limited; and 3) that below this level, there are good reasons to believe that the disease should gradually die out.
MDT has met with considerable success in drastically reducing prevalence over the last fifteen years. However, the use of preventive measures could become relevant in the future under two circumstances: 1) if, in the long term, achievement of the desired reduction of prevalence was found not to result in the expected curtailment of transmission; and 2) if, for some reason, one or the other of the three premises on which the elimination program rests were called into question.
Regarding reduction of the transmission of M. leprae, it is still too early to draw firm conclusions. However, the large and increasing number of newly detected cases, reported in some countries in recent years in spite of considerable reduction of the prevalence rates, is a source of growing concern. Globally, some 750,000 cases were detected in 1998. No doubt, there are many epidemiological or operational reasons at hand to explain this phenomenon.
Because of the long delay between infection and onset of the clinically recognizable disease, it could be that numbers of patients who were infected before the advent of MDT continue to appear as new cases. It is indeed known that some patients may develop the disease many years after having been infected. However, the proportion of patients with a latency period 10 or 15 or more years in duration is likely to be minimal, and, by now, it should markedly decrease with time.
Concurrently, it has been noted that a large proportion of these newly registered cases is actually old cases who, for one reason or another, have previously eluded detection. In 1995, the estimated ratio of the total number of cases to the number of cases registered in the 19 countries with the highest prevalence varied from 1.1 to 9.1. A considerable number of old cases have been detected. Provided current efforts at early detection are sustained, the proportion of late diagnosis among the newly detected cases should rapidly decline.
Expanding coverage of MDT, which is also invoked, cannot account for the recent increase of new-case detection because, according to WHO, treatment of the registered cases now reaches over 99 percent of the estimated endemic areas worldwide.
Finally, could the increase be attributed to a modification of the case definition of leprosy in the direction of greater sensitivity? The definition of a case of leprosy has been modified in order systematically to include single macules, which, in some countries, characterize 30 percent of the new cases. Even if the case definition had not been changed, the very fact of distributing single-dose "blister packs" for these cases has made their detection more likely than it was in the past. This explanation is relevant mostly to India, which accounts for some two-thirds of the cases detected during the last few years.
The interpretation of the detection figures is therefore ambiguous, for there is no clear way to make the distinction between old cases detected late and the new cases that represent the actual incidence.
The continuing large numbers of new cases detected are definitely a source of worry. There is concern that prevalence is not the reliable predictor of future incidence, as it was assumed to be. If so, the first basic premise of the elimination initiative, that by treating and curing the patients transmission of M. leprae would be reduced and, in the long term, interrupted, is open to question. The reason could be that humans are not the exclusive reservoir of M. leprae, or that the clinically recognizable patients are not the sole source of infection. Several hypotheses could be put forward, such as the existence of an extra-human reservoir, in animals, in vegetation, or in the soil, or that individuals with subclinical infections play a role in transmission. These hypotheses are part of another debate. However, they emphasize the possible relevance of preventive measures in the future. A simpler explanation is that patients with very recent onset of disease play a larger than expected role in transmission, however short the delay before detection.
Turning to the second premise, which assumes that, at a prevalence of 1 per 10,000, the potential for transmission is close to nil, setting such an arbitrary target is a most efficient tool, from the standpoint of management. From a rigorous epidemiological standpoint, however, the target is valid only inasmuch as the risk of becoming infected is evenly distributed in the population, i.e., the prevalence is homogeneous. However, this is seldom the case. The prevalence of leprosy most generally resembles a patchwork-high in one place, and low in the vicinity. This results in paradoxes. Leprosy may be declared eliminated in a large country with thousands of registered patients scattered over wide areas; whereas it will defy elimination in a small country, with half a dozen patients above the critical prevalence making the difference. Examples of such a situation may be found in the Western Pacific region. To offset this effect, it is now proposed to scale down the population denominator to smaller sizes, from global and worldwide, as implicitly stated in the WHA Resolution, to country, as in the WHO statistics, and now to subnational administrative or geographical units. Such a procedure will not absorb the heterogeneity, but will only lead to the ceaseless repetition of a pattern of self-similarity, with a succession of foci of higher prevalence concentrating fewer and fewer cases engulfed in larger and larger areas of prevalence below the threshold, in which more and more patients will be dispersed. Pushing the process to the extreme, the time may come when single patients with their retinue of contacts would be the ultimate targets of the elimination campaign. We will have made a complete circle, returning to the abandoned strategy of restricting detection to the contacts, a strategy that, as we know, overlooks the majority of new cases. It is clear that, at some point in this deceptive pursuit of an ever elusive prevalence, preventive measures directed at foci or clusters of patients could prove more cost-effective for reducing the incidence of leprosy than a selective MDT strategy aimed at uncovering widely scattered patients.
Now, let us not confuse the issues. A different strategy should not be advocated, any more than MDT should be discredited, solely because prevalence is a delusive indicator for monitoring the closing-in end-course of MDT. My point is simply that the fetishism of the prevalence target may make us unable to see the forest for the trees, preventing us from realizing the full potential of innovative approaches, such as chemoprophylaxis or vaccination.
The third premise underlying the ongoing elimination program postulates that, upon reaching very low levels of prevalence as a result of MDT, the disease should gradually fade away, replicating the pattern of recession allegedly observed in some countries. Several instances of a decline of the number of patients, leading eventually to extinction of the disease, have indeed been reported in the past. This has occurred in most European countries in modern times, the best documented example being Norway in the second half of the 19th century, as well as among immigrant groups in the Americas.
Various reasons have been offered to explain these observations, among them the improvement of unspecified socioeconomic conditions or a protective immunity against M. leprae, presumably conferred by increasing exposure to M. tuberculosis, associated with the spread of tuberculosis, or exposure to other mycobacteria. These hypotheses open fascinating avenues for speculation and research. They will not be discussed here.
There is, however, a more immediate epidemiological issue, that calls into question the long-term sustainability of the elimination program. Low prevalence, however it is defined, refers to two contrasting situations: "natural low prevalence" on the one hand, in those areas from which leprosy has been disappearing for many decades, and, on the other hand, "induced low prevalence," resulting from MDT. The two dynamics are fundamentally different, as depicted in The Figure. Untreated leprosy "aw naturel" is a life-long ailment. Only after some delay, reflecting the expectancy of life and death rates of the patients, will a decline of prevalence follow a decline of incidence. On the other hand, leprosy treated with MDT is a relatively short disease, the duration of which does not by definition exceed the two years required by the standard treatment. The decline of prevalence, resulting from discharging the cured patients, will therefore precede the decline of incidence. To put it in another way, under natural conditions, the decline of prevalence is the long delayed effect of the interruption, for whatever reasons, of the transmission of M. leprae. When MDT is employed to control leprosy, the decline of prevalence is expected to produce the interruption of transmission, notwithstanding any other factor that could be operating in the same direction. It is, therefore, deceptive to expect that the dynamics of the engineered decline will, at low levels of prevalence, be automatically transformed into the dynamics of the natural decline.
The figure. Schematic of the decline of leprosy prevalence (P) and incidence (I), as it has occurred without intervention in Western Europe and elsewhere (upper panel), and as the prevalence has declined as the result of implementation of MDT , with discharge of the patients from the leprosy register after completion of the course of treatment (lower panel).
From these considerations, it appears that there will be room for preventive measures in leprosy control in the future. There is every hope that the elimination program as presently conducted will run its course to complete success. The next few years will provide a crucial test to validate some of the admittedly questionable assumptions regarding transmission. Yet, it is not too early to prepare for any unpleasant surprise. The development of preventive measures, based on enabling research, is a step toward such preparation.
In the meantime, prevention could be envisaged as a complement to chemotherapy in well circumscribed and preferably small-sized areas. Under these circumstances, incidence will be the appropriate indicator by which to monitor transmission. Provided preventive measures are implemented in the entire population at risk, one should aim at eradication rather than elimination.
However, it should be remembered that the development of preventive measures of proven effectiveness is one issue. Definition of the populations in which these measures are to be applied, and how and under what conditions they should be applied, is another problem, which calls for operational research. In that respect, the chemoprophylaxis programs undertaken in the three countries of the Western Pacific could provide information of major importance for the future.
DISCUSSION
Prof. Ji: Prof. Lechat, would you please summarize your message?
Prof. Lechat: I believe the key point is that we must not allow ourselves to be blinded by MDT to the possibilities of complementary and alternative approaches to achieving the control of leprosy. My second point is that there is a serious lack of important knowledge with respect to the prophylaxis of leprosy, with the result that we are unable to plan strategies for chemo- and immunoprophylaxis.
Dr. Noordeen: Prof. Lechat has raised some very interesting issues. Traditionally, medicine has advanced on the basis of solid information gained from well-controlled research. Methods are not applied in the field until all of the outstanding questions have been answered by research. However, leprosy has been an exception. Beginning in the early 1980s, leprosy control has been ahead of research in the sense that solutions were recommended and applied before all of the evidence supporting the efficacy of the proposed solutions had been obtained. The prime example of this is MDT. If we had waited until all of the necessary proof had been collected, we would be today in the same situation in which we found ourselves in the early 1980s. Admittedly, we took a chance; we could have made a wrong decision, but we took the decision on the basis of the collective judgment of experts. We cannot afford to wait a long time, while the necessary information is accumulated, before we act. But we should take the necessary steps to insure that the information will be obtained at the same time that we decide to act.
With respect to another, more specific issue, Prof. Lechat may have given the impression that he was advocating prophylaxis as a substitute for MDT. In fact, the identification of areas and population groups, in which a relative failure is perceived that requires prophylaxis, depends upon the gathering of solid information, a process that goes hand-in-hand with administration of MDT. Moreover, I am not willing to abandon the individual at high risk of leprosy because we cannot justify prophylaxis on public-health grounds; there is no question that the individual can benefit from prophylaxis, and this potential benefit should not be neglected.
SUBCLINICAL INFECTION BY MYCOBACTERIUM LEPRAE
S. Izumi
National Leprosarium Oshima Seisho-En, Aji-cho, Kagawa, Japan
Since the early 1990s, our research group has been conducting a series of epidemiological studies of leprosy in endemic pockets of Indonesia, the purposes of which were to attempt to explain why leprosy is so endemic in the area, and to collect epidemiological data that will be useful both for the global elimination of leprosy and for preventing the disease.
According to the World Health Organization (WHO), Indonesia is third highest on the list of the most leprosy-endemic countries in the world. In 1999, the number of registered cases is 29,225, with a prevalence rate of 1.41 per 10,000 population. In 1998, there were 15,337 new cases, yielding a new-case detection rate of 7.42 per 100,000 population. Leprosy is heterogeneously distributed in the country; the most endemic province is Maluku, and the majority of the patients in the province reside in North Maluku District, in the northern part of the province. We began a cohort study in the district in 1996, and have since conducted a series of epidemiologic surveys, employing serological and molecular techniques.
More than half of the healthy villagers demonstrated anti-mycobacterial antibodies, and about one-quarter of them appear to carry DNA molecules specific to Mycobacterium leprae on the surface of the nasal mucosa. We interpret these data to indicate the presence of a considerable number of M. leprae in the environment.
MATERIALS AND METHODS
Three typical agricultural villages- Gamtala, Toboso, and Lolori-in North Maluku District were selected. Some demographic characteristics of the villagers are summarized in Table 1. In February 1997, the population of these three villages totaled 1417, and the mean age was 26 years. Villagers ranging in age from 5 to 60 years were selected for this study. To learn the incidence of leprosy and the predictive value of several tests, we surveyed the population of the villages twice-in February 1997 and October 1998.

Villagers were examined dermatologically by three well-trained Indonesian and Japanese specialists. Leprosy patients were classified as paucibacillary (PB) or multi-bacillary (MB) according to the criteria of the WHO.
One ml of venous blood was collected, and the serum, with NaN3 added as a preservative, was stored at -30ºC until used. IgG and IgM anti-PGL-I antibodies and IgG anti-LAM-B antibodies were measured by indirect ELISA. IgM anti-PGL-I antibodies were also measured by a gelatin-particle agglutination test, using the Serodia-Leprae kit.
To examine the possibility that M. leprae in the environment of an endemic area play an important role in M. leprae infection of the populace, we employed a sensitive and specific technique-the nasal swab polymerase chain reaction (PCR) test. The surface of the nasal mucous membrane was swabbed with a wet, sterile cotton swab, and the material adhering to the cotton was removed by washing the swab in phosphate buffered saline (PBS) with Tween80®. The resulting suspension was centrifuged, and the sediment was treated by a lysis buffer containing proteinase-K to obtain the DNA template. An M. leprae -specific DNA fragment was then amplified by a minor modification of Plikytis' nested primer method.
RESULTS
In the course of the first survey, 936 villagers were examined and 24 new cases of leprosy-18 PB and 6 MB-were detected. Including the cases already registered, there was a total of 35 patients, yielding a prevalence of 3.7 percent. In the second survey, 861 villagers were examined, and 21 patients, 15 PB and 6 MB, were detected, yielding a prevalence of 2.4 percent (Table 2).
The incidence of leprosy in the villages was calculated by using the 644 villagers who attended both surveys as the denominator. As is also shown in Table 2, 13 healthy villagers developed leprosy during the 20 months between the two surveys, yielding an annual incidence of 1211 per 100,000 population at risk. Of the 1417 inhabitants of the villages, 342 were household contacts of patients with leprosy, suggesting that the disease affects an important proportion of the households in this area (Table 1).
Of the 885 healthy villagers studied, 304 (34.3 percent) demonstrated anti-PGL-I antibodies at the time of the first survey, and 31.4 percent were found to be seropositive at the time of the second survey. Moreover, it was found that the prevalence of antibodies did not change during the period of 20 months (Table 3). Of the populace, 54.1 percent demonstrated antibodies to PGL-I or LAM-B.
Of 890 swab samples examined, 237 were positive by PCR. This may suggest that a large number of M. leprae are floating in the air of an endemic pocket, which the people in the area breathe (Table 4).
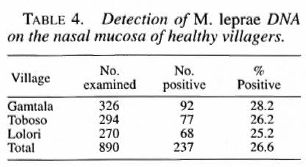
Thirteen new cases were discovered in the course of the second survey. It was found that 11 of the 13 demonstrated at least one leprosy-related factor: six patients were household contacts; six were seropositive; three had suspicious skin lesions during the first survey; and one had an enlarged nerve at that time. However, not one of these factors appears to be useful for predicting who will develop clinical disease.
DISCUSSION AND CONCLUSIONS
One of the most important unsolved questions in the epidemiology of leprosy is the heterogeneous distribution of the disease. Even in an endemic country, there are highly endemic areas-"pockets" or "hot spots." As a result of a series of epidemiological studies in North Maluku, we found the following: 1) a considerable proportion of the healthy residents of the hot spot, who are not household contacts of leprosy patients, appears to be infected with M. leprae; 2) M. leprae appear to be ubiquitous in the environment of the hot spot, and it appears reasonable to assume these environmental organisms play an important role in the infection; 3) development of new immunological tools that may be used to predict who among those at risk will develop clinical disease is one of the most important subjects of research in the future; 4) chemoprophylaxis delivered to those at high risk of developing overt disease is essential for the control of leprosy in the hot spot.
DISCUSSION
Dr. Diletto: It appears that the population of the three villages was 1417, and that you were able to calculate the incidence based on only 644 (approximately 45 percent) individuals who were examined in both surveys. How do you explain the fact that only a relatively small proportion of the population was examined twice? And isn't there some risk that the 644 who were examined twice are different from the majority, who were examined only once, i.e., they are more or less likely to have leprosy?
Dr. Izumi: We examined all of the villagers available to us; we did not select a random sample.
Prof. Ji: As you mentioned, a proportion of tuberculosis patients are also seropositive with respect to LAM-B. Why, therefore, do you consider seropositivity to this antigen as indicative of subclinical infection with M. leprae"? My second question-what proportion of the leprosy patients-both PB and MB-are seropositive or demonstrate M. leprae DNA on the nasal mucosa? My third question has to do with the nature of the acid-fast bacilli (AFB), the DNA of which you find on the nasal swab? There have been a number of published articles, especially in the Japanese literature, regarding the finding of AFB in healthy subjects. I remember that several groups, including that of Prof. Nishimura, published a series of papers in La Lepra in the 1960s. Have you performed nasal swab PCR tests among Japanese in Japan? Finally, do seropositivity and the demonstration of M. leprae DNA persist during and after MDT?
Dr. Izumi: The prevalence of tuberculosis in this population is about 1 percent; therefore, some tuberculosis patients may have been included among the villagers who are seropositive to LAM-B. However, neither infection with M. tuberculosis nor BCG vaccination produces seropositivity to this antigen.
The demonstration of AFB in the nose is quite common, both in Japan and elsewhere. However, we employed as a primer a DNA sequence that is specific to M. leprae, so that PCR positivity cannot be attributed to another mycobacterial species.
With respect to our findings among leprosy patients, one-third of PB patients and 80 percent of MB patients are seropositive. That two-thirds of the PB patients do not demonstrate anti-PGL-I antibodies does not necessarily mean that, during subclinical infection and the incubation period, the antibody titer should be lower than that of the PB patient. We have no evidence to support this hypothesis, but it appears possible that, during the incubation period, M. leprae multiply to a certain level, at which the organisms interact with the host's immune system. When a Thl-type of reaction is triggered, the antigen is quickly destroyed, and the antibody titer falls.
PCR positivity does not at all correlate with the antibody titer, nor does it differ between household contacts and noncontacts. The swab samples only those organisms present on the surface of the nasal mucosa, and does not reach those within the tissues. Organisms could be present on one day and not on the following day; the presence of M. leprae on the nasal mucosa is not related to whether or not the subject is a leprosy patient but, rather, indicates the presence in the air of the organisms, which are then concentrated on the nasal mucosa. Moreover, PCR is unable to discriminate between living and dead organisms.
Prof. Levy: There are techniques, such as air centrifuges, available for air sampling. Wouldn't these represent a more direct means of demonstrating the presence of organisms in the atmosphere? The necessary equipment could not be very expensive. Would it not be important to confirm the presence of M. leprae in the air we breathe? You appear to be using the villagers as sampling devices, and there are more precise and more efficient devices. One could even employ guinea-pigs, as was done for studies of air transmission of M. tuberculosis.
Prof. Smith: It is interesting that you don't find a difference of PCR positivity between household contacts and noncontacts. The work that was done in Sulawesi produced results similar to yours, in that no relationship was demonstrated between seropositivity and PCR positivity; these workers sampled only the anterior portion of the nasal cavity. We've been carrying out similar studies in Ethiopia and India, but we have been sampling the posterior portion of the nasal cavity; these studies have demonstrated a threefold difference between household contacts and the general population. However, a much smaller proportion-only 3-4 percent-of the population has been found PCR positive in our studies.
Dr. Izumi: I do not think our finding that PCR positivity is frequent can be explained on the basis of a difference of the cut-off point. After amplification, we perform electrophoresis on 2% agarose gel, and determine the presence of the specific amplification product simply by the naked eye. We always run positive and negative controls on the same gel. The lower limit of the sensitivity of our system is about 100 M. leprae.
Dr. Kyaw Tin: I wish to return to the question of the size of the bacterial population during subclinical infection with M. leprae. Dr. Ji stated that this is no greater than 106 organisms.
However, it appears to me that someone who will manifest MB leprosy in the future will, at some time before the appearance of his first symptoms, harbor > 106 organisms.
Prof. Ji: This may be so. On the other hand, we believe that the great majority of subclinical infections subside spontaneously, and the aim of chemoprophylaxis is to increase the likelihood that the subclinical infection will subside and not progress to clinical disease.
Dr. Noordeen: One of the problems is the borderline between incubating, subclinical MB leprosy and clinical MB leprosy. People have talked about skin-smear positive, asymptomatic carriers of M. leprae. This is a contradiction in terms; one who is skin-smear positive no longer has a subclinical infection but, rather, overt disease.
Prof. Levy: I have just recalled some data from a study of air-transmission of M. tuberculosis done many years ago in Baltimore. It was found, if I remember correctly, that the average patient with untreated pulmonary tuberculosis produces a concentration of organisms in the air around him of 1 "infectious particle"-one viable organism, or one clump of organisms containing at least one viable-per 13,000 ft3 of air. This concentration appears to me many orders of magnitude smaller than 100 organisms on a nasal swab, even if the indvidual has been breathing the contaminated air for a long time. Again, Dr. Izumi, I believe that your data must be confirmed by some more direct means. It may be that the villager is not a very efficient sampling device and, if you find 100 or more organisms on a nasal swab, it may be that the entire community is living in a cloud of organisms or in the midst of an aerosol. In that case, it is surprising that anyone escapes infection. Your findings may be very important. But I believe you have jumped over many intermediate steps in reaching the conclusions you presented.
Dr. Izumi: Perhaps I did not speak clearly enough. We use only about 5 percent of the sample in the PCR, so a minimum of 100 organisms for a positive PCR test actually indicates a minimum of 2000 organisms in the sample.
Prof. Levy: This statement strengthens my argument. By PCR, you detect bits of DNA. I think it would be very important to confìrm the presence in the air of the viable M. leprne that you infer are present.
Dr. Noordeen: A control might be a nasal swab from your own nose.
Dr. Izumi: We intend to do this.
PLENARY SESSION ON THE NEEDS AND OPPORTUNITIES FOR PREVENTION OF LEPROSY: DISCUSSION, CONCLUSIONS AND RECOMMENDATIONS
S. K. Noordeen
Chairman
Dr. Noordeen: Our task is to review and evaluate the studies in chemoprophylaxis and immunoprophylaxis, and to attempt to reach a consensus on their potential usefulness and application. We have heard presentations of the structure and results of the various studies that have been undertaken, and have considered how to interpret the available data, what further information is needed, and what is the potential usefulness of the means of prophylaxis studied. We should also consider the possible needs for prophylaxis and the available opportunities, and to what degree the studies meet the needs and exploit the opportunities. I propose that we consider separately the studies of chemo- and immunoprophylaxis.
Considering first chemoprophylaxis, can we agree on the needs and opportunities? Certainly one of the reasons justifying chemoprophylaxis is a population at high risk of leprosy. Such a population is represented by the household contacts of leprosy patients. Another reason is the need to achieve the target of elimination, as defined by the World Health Assembly-prevalence at the national level no greater than 1 per 10,000 by the year 2000. This is the reason for the use of chemoprophylaxis in the Federated States of Micronesia (FSM), where it was believed that the elimination target could not be reached unless chemoprophylaxis was added to the on-going program of multidrug therapy (MDT).
Prof. Smith: I think we should not restrict our discussion to areas in which the elimination target has not been reached.
Dr. Noordeen: Prof. Lechat stated in his presentation that new-case detection rates are not falling. Does this justify chemoprophylaxis?
Prof. Lechat: I didn't state that detection rates are not falling. I said that there is general concern but, in fact, we do not know what has been happening. There is no way to determine whether the "newly detected" cases are, in fact, new cases or old "backlog" or "missed" cases.
Dr. Noordeen: Is the need for chemoprophylaxis related to a population at high risk? That is, when new-case detection rates remain high, shouldn't one add chemoprophylaxis?
Prof. Lechat: I believe that chemoprophylaxis is very interesting in some special situations-geographically closed area, isolated population, situations in which there is a small denominator with foci of high incidence. I'm concerned that, because we are so determined to reduce the prevalence in order to meet the elimination target, we risk concentrating our efforts more and more intensively on smaller and smaller foci of high prevalence, at the same time forgetting the much larger number of patients who are to be found in the much larger areas of low prevalence.
Prof. Levy: I think that we are struggling with the possibility of failure of MDT. For whatever reason, we have not succeeded in reducing the detection rate, so we begin to believe that something additional is needed, as was the case in the FSM.
Dr. Noordeen: The primary purpose of MDT was to reduce prevalence, and we assumed that detection rates would diminish in time. If the reduction of detection rates requires more time than we had originally assumed, then a second intervention is required, e.g., chemoprophylaxis.
Prof. Lechat: Something additional is needed when prevalence decreases below the rate of new-case detection, at which point prevalence ceases to have any meaning. In fact, chemoprophylaxis may be expected to have an effect on the new-case detection rate, but can have very minimal effect on prevalence.
Dr. Gupte: One can think of situations in which leprosy might complicate other preexisting conditions, such as pregnancy. A pregnant woman who manifests leprosy for the first time during pregnancy is very likely to have reactions, and one would wish to prevent these. Might the pregnant woman who is a household contact not represent a need for chemoprophylaxis?
Dr. Crippen (observer from the Centers for Disease Control, USPHS, Atlanta, Georgia, U.S.A.): With respect to needs, would it not be worthwhile to review the existing data gained from the three programs of chemoprophylaxis here in the Western Pacific in an attempt to determine their efficacy and arrive at a standard protocol?
Dr. Noordeen: Certainly, there remains a need to evaluate the data relating to available drugs and regimens that have been produced in these three programs and, based on this analysis, to attempt to define the populations in which chemoprophylaxis should be used and the strategies by which it should be employed.
So much for the needs for chemoprophylaxis. May we now consider the opportunities for chemoprophylaxis and the constraints on its use? I suggest that heading the list of "opportunities" should be the availability of effective drugs. And the first item on the list of "constraints" should be the lack of solid data attesting to the efficacy of chemoprophylaxis.
Prof. Lechat: We should consider the cost-effectiveness of the two alternative approaches-administration of chemoprophylaxis to individual contacts or to an entire population.
Dr. Noordeen: Certainly, cost of the drugs and their administration must be considered constraints.
Dr. Blanc: Among the constraints should be listed the lack of important epidemiological data.
Prof. Ji: Hyperendemic pockets of leprosy often coincide with a very weak infrastructure of health services. As a result, it could be very difficult to organize and conduct a program of chemoprophylaxis just where it is most needed. I also should mention that there are two basic requirements for an area to be suitable for a program of chemoprophylaxis: 1) all of the known patients must be treated or have been treated by MDT and 2) leprosy patients must be absolutely excluded from the chemoprophylaxis. In turn, both of these requirements demand some infrastructure.
Dr. Diletto: I was pleased to learn from Prof. Ji's paper that there is a firm experimental basis for the decision to employ single doses of rifampin (RMP) or the combination rifampin, ofloxacin and minocycline (ROM). This points up the need to confirm the efficacy of single doses of RMP or ROM as chemoprophylaxis. Thus, perhaps the emphasis should be placed on the need to confirm the efficacy of the drugs in a trial, rather than simply to assume their efficacy and consider the availability of drugs an opportunity.
Prof. Levy: On the one hand, to establish the efficacy of a drug or a regimen is not a simple task. On the other hand, studies of ROM are currently in progress, and much data have already been collected. In addition, we already know a great deal about the efficacy of single doses of RMP. We face much the same sort of problem as was faced by the WHO Study Group in 1981. Then, there was an urgent problem of increasing dapsone resistance and a chaotic situation, in which individual leprosy-control programs were adopting a variety of multidrug regimens, some of which appeared to lack efficacy and encourage the spread of RMP resistance. This Workshop was convened to help deal with another urgent situation-continuing high new-case detection rates despite MDT-that threatened to get out of hand. Although studies would be useful, they will require a great deal of time; however, we already have some basic information that can be exploited.
Prof. Lechat: It appears that we have reached a threshold as the prevalence of leprosy falls below the detection rate. This means that we treat and cure the patients as they appear, but that we may not be making much progress in the realms of control or eradication. At this point, we must begin to consider approaches in addition to MDT.
Dr. Noordeen: I believe that your argument depends upon the level of the new-case detection. If the detection rate were higher than the prevalence, but as low as 0.01 per 10,000, one would not advocate prophylaxis. Certainly, we need good data on incidence; even this may be only an unreachable ideal, except in situations like that in the FSM in which it should be possible to measure the incidence, and not be forced to rely on raw case-detection data.
Dr. Blanc: I don't believe we need data on true incidence in order to justify a program of chemoprophylaxis; the case-detection rate should suffice.
Dr. Noordeen: Must we not consider the acceptability of chemoprophylaxis? This is not a matter of ethics, but rather whether healthy people-members of the general population, or household contacts-will accept a chemoprophylactic regimen, regardless of the drugs included.
Prof. Smith: One must also consider the risk of adverse reactions. In Micronesia, 150,000 doses of ROM have been administered, thus far without a serious adverse reaction. It appears likely indeed that such reactions will be encountered and, as a result, the regimen will become much less acceptable.
Prof. Lechat: I think we have not addressed another important issue. There are many rare diseases in the world, of which leprosy is only one. Are we justified in attempting to eradicate leprosy? I feel ashamed when I recognize how much money is spent on leprosy, whereas virtually nothing is spent on other diseases that are more important, at least locally. For example, I think of sleeping sickness in Africa. Considering that, last year, there were 5000 deaths from sleeping sickness in the Congo alone, it appears unjustifiable to run after the last case of leprosy.
Prof. Levy: Would it not be appropriate to discuss the trials in Micronesia in greater detail? For example, during the several papers on the program here in the FSM, I found myself wondering what the results would be of one more round of screening.
Dr. Blanc: One of the needs that has been expressed is that relating to evaluation of the data from the programs in the three Western Pacific countries in which entire populations have been screened and, in some, chemoprophylaxis administered. I had hoped that a result of this Workshop would be the recommendations of the experts assembled here.
Prof. Lechat: The purpose of this Workshop, as I understand it, is not so much to evaluate data, as from a clinical trial, but rather to attempt to interpret the data in a way that will enable us to define a strategy for chemoprophylaxis interventions.
Dr. Noordeen: May we conclude that the data presented here justify such an intervention?
Prof. Levy: I think that the data clearly justify the activity surrounding the administration of chemoprophylaxis. Although it may not be clear that chemoprophylaxis was effective, there is no doubt that the intensive screening led to the discovery of a great many new cases.
Dr. Noordeen: There is a parallel in MDT. Implementation of MDT resulted in much improved treatment of patients. In addition, there were many indirect benefits- improved case detection, better case holding, better organization of leprosy-control activities, and diminished stigma. These were not intended, but they would not have occurred without MDT. In the same way, chemoprophylaxis brings with it detection of backlog cases, improved case holding, and MDT treatment of patients.
Prof. Smith: Surely you are not arguing that this justifies chemoprophylaxis everywhere.
Dr. Noordeen: You're correct. These are indirect benefits, but not the purpose of chemoprophylaxis.
Prof. Ji: One could achieve the same goals by means of a leprosy elimination campaign (LEC), without prophylaxis.
Prof. Levy: I agree, but if one is already engaged in mass screening, why not also administer a dose of RMP?
Prof. Ji: Do we have enough evidence to justify the chemoprophylaxis?
Prof. Levy: That was the point I made earlier-we can't wait to accumulate the evidence. Ten years, more or less, will be required; why wait?
Prof. Lechat: I agree with Prof. Ji. If efficacy has not been proven, and if there is some risk of serious side effects, I think it would be unethical to embark upon such a program.
Prof. Ji: For this reason, I support Prof. Levy's suggestion that an additional round of screening be carried out. This may be the only opportunity to obtain evidence of efficacy of the chemoprophylaxis.
Dr. Blanc: We already have good evidence of the efficacy of ROM.
Prof. Ji: I agree and I disagree. Certainly ROM is active in treating leprosy. However, we certainly do not know whether a dose of ROM is capable of preventing the disease. Another round of screening in the FSM after two or three years might represent the only opportunity to obtain data regarding the efficacy of ROM chemoprophylaxis. And it would not be necessary to wait an additional ten years.
Dr. Noordeen: Even assuming the chemoprophylaxis to be effective, could it have a global or national impact? It appears clear that chemoprophylaxis could be employed only in a more localized fashion, and have only a local impact. With respect to MDT, we believe that every patient everywhere should be treated with MDT. We do not believe that every individual at high risk of leprosy should be administered chemoprophylaxis, but that chemoprophylaxis should be employed only in certain specific situations. A question that might be raised is how should one define "high risk"?
Dr. Gupte: I suggest that any population in which the new-case detection rate is 1 per 10,000 or greater might be defined as a high-risk population.
Prof. Smith: Such a low rate would result in the treatment of enormous numbers of people. I suggest that the minimal rate be 10 per 1000. Although there are very few communities with so high a case-detection rate, consider the "numbers necessary to treat" (NNT). One can't consider treating thousands of people to prevent one case. Employing a chemoprophylaxis that is 100 percent effective, one would need to treat 1000 to prevent one case, if the case detection rate were 1 per 1000.1 believe that the risk of serious side effects would prohibit chemoprophylaxis in such a situation.
Prof. Ji: For the purpose of comparison, chemoprophylaxis against MAC infection has a ratio of 6:1, i.e., one must treat only six people to prevent one case.
Dr. Noordeen: We could not envision a similar ratio with respect to leprosy. On the other hand, Prof. Smith's suggested figure of 10 per 1000-one percent-reflects the situation among household contacts.
Prof. Smith: Remember that a detection rate of 1 per 1000 requires the treatment of 1000 people to prevent one case. With respect to the FSM, certainly within the population are foci in which the detection rate approaches one percent. Perhaps chemoprophylaxis should have been restricted to these foci.
Dr. Blanc: If we limit our activity to very high-risk groups within a population at relatively high risk, aren't we returning to the situation exemplified by the trial of acedapsone in Pingelap? There was an impact on the case-detection rate, but it was limited to only two years because, it was believed, the population was not protected against the risk of infection from outside the very high-risk group.
Dr. Diletto: Upon review of the villageby-village data in Pohnpei and Chuuk States, it is clear that there are indeed villages in which the case-detection rate exceeded 1 percent.
Prof. Lechat: The denominator, consisting of those who have been administered chemoprophylaxis, includes would-be patients who are infected but who probably cannot transmit the organism. On the other hand, we know that the patients who are not detected and are not treated for some period can definitely transmit the organism. Therefore, is it not reasonable, because one must screen the population in any case, to detect the cases as early as possible and administer MDT, so as to render them noninfectious, and thus prevent transmission? This is cheaper, more effective and absolutely safe. This is in fact what is done in a LEC.
Dr. Noordeen: The bulk of the cost of a program of chemoprophylaxis results from the screening, which is essential to the program; the drugs are costly, but do not account for a large fraction of the cost of the program. Where does one go from here? I think that one should weigh the cost of screening, the cost of the single-dose chemoprophylaxis, the resources available, and the perception of what represents high risk.
Dr. Blanc: There is a problem in the identification of high-risk foci because, in fact, one must depend for this upon passive case detection. It is only after one is aware of the existence of high-risk foci that one plans a program of screening and chemoprophylaxis.
Dr. Noordeen: Can we proceed with programs of chemoprophylaxis without first obtaining additional data with respect to efficacy? To this moment, we have depended upon experimental data attesting to the efficacy of the drugs against M. leprae, and the effectiveness of the drugs in the treatment of patients. In addition, a few studies have demonstrated the efficacy of chemoprophylaxis with dapsone and acedapsone. Do the available data confirm the efficacy of ROM chemoprophylaxis?
Prof. Smith: The available data are not adequate, and we require a randomized controlled trial of chemoprophylaxis to demonstrate efficacy.
Prof. Levy: Why don't we examine the available data more closely?
Dr. Noordeen: But a controlled trial will be very demanding. Does any of us believe that the available data should permit some reasonable conclusion?
Dr. Gupte: I believe that a careful analysis of the available data may lead to some useful conclusions.
Prof. Lechat: We have collected a considerable quantity of data in these three countries, and it would be a serious error not to exploit this opportunity to the fullest. However, it appears likely that such an effort will not provide an unequivocal answer, and we will be left with the necessity to mount a formal controlled trial in order to demonstrate efficacy.
Prof. Ji: Because of the lack of a control group, I don't believe that it will it be possible to determine the efficacy of ROM chemoprophylaxis from the work -in the FSM, RMI and Kiribati.
Dr. Noordeen: It is my impression that the consensus of this group is that, before deciding upon a formal controlled trial, we examine the available data more closely and conduct a third round of screening.
Prof. Smith: I believe with Prof. Ji that, because these programs were not designed for this purpose, we will not be able to state with confidence that chemoprophylaxis was or was not effective. We should certainly examine the available data more closely and conduct another round of screening, but these activities will not serve as a substitute for a formal trial.
Dr. Blanc: I agree with Prof. Smith. The three programs in the Western Pacific were designed to assist in control of the disease in the face of high endemicity; there was never any intention to measure the efficacy of the chemoprophylaxis. It was assumed, on the basis of experimental evidence, that ROM was an effective antimicrobial combination, and the results of the trial in the Marquesas were taken as evidence that a single dose of RMP was by itself effective as chemoprophylaxis. A third round of screening and a closer examination of the data should provide some measure of efficacy.
Dr. Noordeen: When one introduces a new drug or regimen into a population, is not one obliged to attempt to determine whether it is effective or not?
Prof. Lechat: It has been said that ROM chemoprophylaxis was introduced to deal with an emergency. What was the nature of the emergency?
Dr. Yuasa: Perhaps "emergency" is too strong a term. There were many new cases and a continuing high prevalence, and we assumed that, unless something were done, there would be many more. It was clear that MDT as it was being applied was not enough. Perhaps it would have been sufficient simply to improve the MDT program; however, based on our 20 years of experience in this part of the world, that degree of improvement appeared unlikely, and it was believed necessary to add something that would produce results in the short term. We took a chance-I believe a reasonable chance. The intention was not to carry out research. On the other hand, we felt that something had to be done, if we were to reach the elimination target. Chemoprophylaxis was the only additional measure we could think of.
Prof. Lechat: Particularly with the history of leprosy in this area, the addition of chemoprophylaxis appears reasonable. The question now is whether we can extrapolate from the experience here.
Prof. Levy: As I recall, there were special circumstances that dictated the addition of chemoprophylaxis. Not only was there a continuing high prevalence, but also there had been discovered during the previous year a large number of children with MB disease, suggesting that there was continuing active transmission despite a reasonable effort to apply MDT.
Prof. Smith: I don't think it's appropriate to second-guess the decision to employ chemoprophylaxis. The important question, as Prof. Lechat has pointed out, is whether we can generalize from the experience here. I believe that we should pursue the obtaining of additional evidence here, but I do not believe that there will result sufficiently robust evidence to permit us to generalize to other situations.
Prof. Lechat: I believe that we should not be satisfied simply with the statement that we should exploit the data from the three Western Pacific programs to the maximum. I believe that we should also prepare a list of those items in which we are most interested. Specifically, what information can be extracted from these programs?
Dr. Noordeen: I believe that we are coming to a consensus. We wish to learn the effect of the chemoprophylaxis in this population. We believe that the information presented is not sufficient to permit firm conclusions. Additional analysis may be required, as well as the collection of additional data. In my opinion, it is not necessary once again to screen the entire population of the FSM, but it would be sufficient to screen the population of selected communities in Pohnpei State. Collecting additional data in outlying islands and sparsely populated areas, such as Yap State, is simply not feasible.
Prof. Lechat: It's not so much a matter of collecting additional data as of making available additional data that have already been collected but have not been included in the reports presented here.
Dr. Noordeen: A third round of screening is already being planned. I suggest that Dr. Blanc appoint an epidemiologist who would analyze as completely as possible the available data, and plan the optimal approach to collecting additional information. It may be possible to establish trends; a controlled trial is ideal, but is not always possible.
Prof. Ji: In the FSM program, 80 new cases were detected in the course of the second round of screening, of whom 12 had been administered chemoprophylaxis during the first round. Thus, 68 new cases arose among those who had not been treated in the first round. Does this information have any value?
Prof. Smith: The information is limited in two ways. First, allocation to the two groups-those treated and those not treated during the first round-was not random, and is likely to have been highly biased. Second, those not treated during the first round were similarly not screened at that time, and the new-case detection rate among them is likely to be the same as that during the first round among those who were treated during the first round. Therefore, a comparison of the two groups is not very meaningful. Again, I wish to point out that, although it will be possible to perform additional analyses, there will remain important limitations to what can be learned.
Prof. Ji: Prof. Smith's points are well-taken. However, compare the figures. Those who had been administered chemoprophylaxis during the first round yielded a case-detection rate of 1.5 per 10,000 during the second round, whereas the rate among those not screened and not treated during the first round was 22 per 10,000. Doesn't this difference suggest that the chemoprophylaxis was efficacious?
Prof. Lechat: This is exactly the sort of question that should be considered by Dr. Blanc together with some experts.
Dr. Noordeen: Can we agree on that, if this is thought necessary? We have agreed upon some needs and opportunities for chemoprophylaxis, particularly among high-risk groups such as household contacts, although we could not define the degree of risk, and that chemoprophylaxis may be useful in achieving the elimination target. Moreover, we have agreed that the data available from the programs in the FSM, RMI and Kiribati should be re-analyzed, and more should be collected, as by a third round of screening. Dr. Diletto or Dr. Blanc, how had you planned to proceed in the FSM?
Dr. Blanc: The plan was to assess the sensitivity of the surveillance mechanism after completion of the chemoprophylaxis program by allowing patients to report to the health center of their own initiative, and then screening the populace of villages of high endemicity to learn how many cases had been missed. It now appears important to make a better assessment of the trend of new-case detection.
Dr. Farrugia: The program in the RMI differed in one important aspect from that in the FSM. In the RMI, chemoprophylaxis has been administered only to household contacts, whereas in the FSM, it was administered to the entire population. Dr. Diletto, among the population administered chemoprophylaxis in the FSM, can you distinguish the household contacts?
Dr. Diletto: No, unfortunately not. Recording was done village-by-village.
Dr. Daulako: In the FSM, the teams generally received the population in some central venue in each village, whereas in Kiribati, although the entire population was screened, the teams went from house to house. As a result, in Kiribati, it will be possible to consider the household contacts apart from the noncontact population.
Dr. Blanc: The data that have been presented thus far from the work in the three countries of the Western Pacific are only preliminary. I'm confident that some of the questions that have been asked could be answered if one were to examine the available data more closely. One task that has not yet been completed is to re-interview all of the patients to ascertain whether their lesions appeared before or only after chemoprophylaxis. I don't believe it will prove a difficult task to review all of the records and extract additional information.
Dr. Gupte: Re-interviewing patients may not be very helpful. One wonders how well the patient will remember if he was'administered a single-dose treatment, and when it was, in relation to the onset of his lesions. Because we wish to observe trends, what will be important is to carry out screening and detect the new patients.
Prof. Smith: An important outcome of the programs in the three countries is the accumulation of valuable experience in implementing chemoprophylaxis. Information regarding the reasons for excluding residents from chemoprophylaxis, and information with respect to adverse reactions is valuable, and should be published. Just think-in the FSM, 150,000 doses of chemoprophylaxis were administered to apparently healthy people.
Dr. Keller: No serious adverse reactions were encountered in Pohnpei State-nothing more serious than urticaria and nausea and vomiting.
Dr. Noordeen: I'd like to call attention to the "individual benefit" of chemoprophylaxis, as distinct from the "public health benefit." There can be no doubt of the benefit of chemoprophylaxis to the individual who is a household contact or a member of a high-risk group, given an effective drug or drug regimen. How would one employ such a chemoprophylaxis?
Prof. Smith: I believe much would depend upon the degree of efficacy. One might employ a chemoprophylaxis that is 100 percent effective differently from one that is only 50 percent effective. One also needs to consider the risk of side effects, the cost and the quantity of resources available. With respect to the benefit to the public health, this will vary with the proportion of the community deemed to be at high risk. If this proportion is very small, the benefit to the population would be similarly small.
Prof. Ji: In designing a trial to measure the efficacy of a chemoprophylaxis, one would need first to decide the minimal level of protection that would be useful. What should be the minimal level; is 50 percent high enough?
Dr. Farrugia: In the Marquesas trial, the level of protection was calculated to be about 50 percent. Cartel thought that this was too low a level to be useful.
Prof. Smith: Much depends upon the level of risk. If the risk is very high, 50 percent protection is worth having. If, on the other hand, the risk is very small, then even 100 percent protection might not be sufficient justification for a program of chemoprophylaxis.
Dr. Gupte: In designing our Indian vaccine trial, we assumed a protective efficacy of 65 percent. The prevalence of leprosy had decreased to a level of about 10 per 10,000, and we calculated that about 10 years would be required to reach the target of 1 per 10,000.
Dr. Noordeen: Although you have shown that a potent vaccine to some extent aborts incubating infections, its primary purpose is to protect the uninfected. On the other hand, chemoprophylaxis aborts incubating infections, so that its effect should be evident much more immediately.
Dr. Izumi: Early case finding is essential to the success of a program of chemoprophylaxis. A continuous monitoring system, which should be most helpful in case finding, requires the active participation of the members of the community.
Prof. Smith: In thinking about the nature of the chemoprophylaxis to be employed, I have a question for Prof. Ji. You have said that the likelihood of side effects is smaller with a one-drug regimen than with a three-drug regimen, and that the risk of selection of drug-resistant mutant M. leprae is extremely small. However, if one extrapolates the bacterial population of the infected individual-approximately 10s-to a community of 1000 infected individuals, is not the bacterial population exposed to the drug now approximately 109 and can you still say that the risk of selection of mutants is extremely small?
Prof. Ji: The total population of M. leprae in the community must certainly be larger than 10''. On the other hand, work published a number of years ago by Dr. Blanc and his colleagues at the Institut Mar-choux in Bamako, Mali, demonstrated that, although single doses of RMP were insufficient to prevent relapse in MB patients, who had become smear-negative on dapsone monotherapy, the single doses were also insufficient to select the RMP-resistant mutants, i.e., none of the relapses was found to be RMP-resistant.
Prof. Levy: I remember a paper by Grosset and his colleagues in which it was shown that the risk of relapse with RMP resistance was related to the number of doses of RMP as monotherapy that the patient had received. Those patients who had relapsed after only a few doses of RMP relapsed with RMP-susceptible organisms. Thus, the risk that a single dose of RMP as chemoprophylaxis will select the RMP-resistant mutants mast be negligible.
Dr. Izumi: What is the risk that a single dose of RMP will select RMP-resistant mutant M. tuberculosis, should an undiagnosed tuberculosis patient inadvertently be administered the chemoprophylaxis?
Prof. Ji: A single dose of RMP has virtually no effect upon a population of M. tuberculosis. M. tuberculosis is much less susceptible to RMP than is M. leprae.
Dr. Izumi: If a single 600-mg dosp of RMP is not capable of selecting the RMP-resistant mutants, does this mean that, after such a dose, RMP-susceptible M. leprae will survive?
Prof. Ji: Yes. This is probably the case for whatever chemoprophylaxis regimen is employed. And among those inapparently infected individuals with exceptionally large bacterial populations, there will be failures of chemoprophylaxis.
Dr. Noordeen: If I may summarize our conclusions with respect to chemoprophylaxis, chemoprophylaxis employing an effective regimen is valid for household contacts and high-risk groups and populations. The open questions have to do with efficacy, the minimal level of risk, side effects, cost and availability of resources, and operational issues-organization of distribution of drugs; acceptability of the program to individuals, populations, and health services; the difficulties of excluding patients with leprosy; and the need concurrently to treat the patients. A formal trial of a single 600mg dose of RMP is recommended to establish evidence of efficacy. Finally, chemoprophylaxis cannot be recommended for program use until the efficacy of a regimen has been established.
May we now turn our attention to immunoprophylaxis? Three vaccines appear to be effective-BCG, BCG+HKML, and the ICRC bacillus.
Prof. Levy: An obvious difficulty is that the combined vaccine will no longer be readily available. The ICRC vaccine would be much more readily available, if we were satisfied that it is efficacious. At least one more formal trial of the ICRC vaccine should be carried out in a setting other than India.
Dr. Matsuo: Is there evidence that any of the vaccines prevents MB leprosy, or that they are capable of converting Mitsudanegatives to reactors?
Dr. Gupte: In the trial of BCG in South India, smear-positive cases were not prevented, but BCG did prevent borderline leprosy. In our trial, we found that Mitsuda reactivity did not correlate well with prevention.
Prof. Smith: It appears likely to me that one could usefully apply a vaccine at a lower level of risk than would be required to justify a program of chemoprophylaxis, because the effect of the immunoprophylaxis endures for a much longer period of time than does that of chemoprophylaxis.
Dr. Gupte: We have evidence that the protection conferred by the two most effective vaccines endures for at least six years.
Dr. Noordeen: Is there evidence that the protective efficacy of BCG against tuberculosis wanes over time?
Dr. Blanc: The protective efficacy of BCG varies very widely from one place to another. In general, the protective efficacy of BCG against tuberculosis lies in the range 30-50 percent. There is general agreement that it prevents the severe forms of tuberculosis-miliary tuberculosis and meningitis-in children.
Dr. Gupte: There is clear evidence that the protective efficacy of BCG against tuberculosis wanes over time.
Prof. Smith: There is an additional benefit to BCG, in that it protects against both leprosy and tuberculosis. On the other hand, the vaccine must be living, so that its distribution requires a cold chain, which may pose operational difficulties.
Dr. Noordeen: The efficacy of chemoprophylaxis depends upon concurrent treatment of patients so that the risk of infection after prophylaxis is diminished. The efficacy of immunoprophylaxis does not depend upon concurrent treatment.
To conclude, many of the open questions that are pertinent to chemoprophylaxis appear also to pertain to immunoprophylaxis, as well as to the possibility of combining chemoprophylaxis and immunoprophylaxis.
CONCLUSIONS AND RECOMMENDATIONS
Chemoprophylaxis employing dapsone or diacetyldapsone has been shown to be efficacious in the individual, and chemoprophylaxis employing rifampin is very likely to be efficacious. Therefore, the members of this Workshop recommend: 1) a more detailed examination of the data from the FSM, RMI and Kiribati and 2) the conduct of a placebo-controlled trial of 600 mg rifampin in a single dose.
Assuming chemoprophylaxis by a single dose of rifampin to be efficacious, this could be recommended for use among household contacts and in very high-risk population groups (i.e., annual incidence >1 per 100).
The points to be considered before the introduction of chemoprophylaxis are: 1) the level of efficacy; 2) the level of risk of disease, and the proportion of the population at high risk; 3) the risk of side effects; 4) operational aspects; and 5) the cost-benefit ratio, and the availability of resources.
Immunoprophylaxis employing BCG has been shown to be efficacious in Africa, and immunoprophylaxis employing BCG plus heat-killed M. leprae or the ICRC bacillus has been shown to be efficacious in South India. Therefore, the members of the Workshop agree that the use of immunoprophylaxis is valid for household contacts and high-risk groups (i.e., annual incidence >1 per 1000).
Points to be considered before introducing immunoprophylaxis are: 1) duration of the protection; 2) the level of efficacy; 3) the level of risk, and the proportion of the population at high risk; 4) the risk of side effects; 5) operational aspects; 6) the cost-benefit ratio, and the availability of resources; and 7) additional, beneficial effects on other diseases (e.g., tuberculosis).
Finally, the use of a combination of chemoprophylaxis and immunoprophylaxis should be considered.
LIST OF WORKSHOP PARTICIPANTS
Dr. Leopold J. Blanc
Regional Adviser in Chronic Communicable Diseases
World Health Organization
Regional Office for the Western Pacific
United Nations Avenue, P.O. Box 2932, 1000 Manila, Philippines
Fax: +63-2-521-1036 e-mail: blancl@who.org.ph
Prof. Patrick. J. Brennan (invited but unable to attend)
Department of Microbiology
School of Veterinary Medicine
Colorado State University
Fort Collins, CO 80523-1677, U.S.A.
Fax: +1-970-491-1815 e-mail: pbrennan@cvmbs.colostate.edu
Prof. Sang-Nae Cho
Department of Microbiology
Yonsei University College of Medicine
134 Shinchon-dong, Seoul 120-752, Korea
Fax: +82-2-392-7088 e-mail: raycho@bubble,yonsei.ac.kr
Dr. E. C. Daulako
PJ Twomey Memorial Hospital
Suva, Fiji
Fax: +679-320836
Dr. Carmine Diletto
c/o World Health Organization
Regional Office for the Western Pacific
United Nations Avenue, P.O. Box 2932, 1000 Manila, Philippines
Fax: +63-2-521-1036 e-mail: dilettoc@who.org.ph
Dr. Roland Farrugia
P.O. Box 1164
Crows Nest
Sydney, NSW 2065, Australia
Fax: +61-2-9817-4525 e-mail: farroland@ozemail.com.au
Prof. Paul E. M. Fine (invited but unable to attend)
Department of Infectious and Tropical Diseases
London School of Hygiene and Tropical Medicine
(University of London)
Keppel Street, London WC1E 7HT, U.K.
Fax: +44-171-436-4230 e-mail: p.fine@Ishtm.ac.uk
Dr. M.D. Gupte
Director
National Institute of Epidemiology
(Indian Council for Medical Research)
Mayor V.R. Ramanathan Road, Chetput, Chennai 600 031, India
Fax: +91-44-826-4963 e-mail: nieicmr@vsnl.com
Dr. Elizabeth Iehsi-Keller
Chief of Staff, Pohnpei State Hospital
Department of Health Services
Department of Health, Education and Social Affairs
Kolonia, Pohnpei, FSM
Fax: +691-320-5394 e-mail: psdhs@mail.fm
Dr. Shinzo Izumi
Chief Surgeon
National Sanatorium, Oshima Seisho-En
6034-1 Aji-cho, Kita-gun, Kagawa 761-0918,Japan
Fax: +81-87-871-4821 e-mail: izumis@oosimasei.hosp.go.jp
Prof. Baohong Ji
Bactériologie et Hygiène
Faculté de Médecine Pitie-Salpêtrière
91, Boulevard de l'Hôpital, 75634 Paris Cedex 13, France
Fax: +33-1-45-82-7577 e-mail: baohong_ji@yahoo.com
Prof. Michel F. Lechat
Université Catholique de Louvain
1200 Brussels, Belgium
Fax: +32-2-673-2476 e-mail: michel-francois.lechat@epid.ucl_ac.be
Prof. Louis Levy
Moshav Shoeva 3
Mobile Post Harei Yehuda
90855 Israel
Fax: +972-2-570-1165 e-mail: deelou@netvision.net.il
Dr. Eiichi Matsuo
Director
Leprosy Research Center
National Institute of Infectious Diseases
4-2-1, Aoba-cho, Higashimurayama-shi, Tokyo 189-0002, Japan
Fax: +81-42-391-8210 e-mail: e-mats@nih.go.jp
Dr. S. K. Noordeen
International Leprosy Association
9 Anna Avenue, B.V. Nagar Extension
Adyar, Chennai 600 020, India
Fax: +91-44-441-0746
Dr. Eliuel K. Pretrick
Secretary, Department of Health Services
Government of the Federated States of Micronesia
P.O. Box PS70
Palikir, Pohnpei, FM 96941
Fax: +691-320-5263 e-mail: fsmhealth@mail.fm
Prof. W. Cairns S. Smith
Department of Public Health
University of Aberdeen
Fosterhill, Aberdeen AB25 2ZD, U. K.
Fax: +44-1224-662994 e-mail: w.c.s.smith@abdn.ac.uk
Dr. Junya Takashima
Department of Surgery
International Medical Center of Japan
1-21-1 Toyama Shinjukku-ku, Tokyo, Japan
Fax: +81-3-3202-7181 e-mail: takashimajr@pop16.odn.ne.jp
Dr. Kyaw Tin
No. 15(a) U Tun Myat Road
Tamwe P.O.
Yangon 11211, Myanmar
Fax: +951-552167 e-mail: myo.paing.whomm@undp.org
Dr. Yo Yuasa
Medical and Executive Director
Sasakawa Memorial Health Foundation
Nippon Zaidan Bldg. 4F
1-15-16 Toranomon, Minato-ku, Tokyo 105-0001, Japan
Fax: +81-3-3508-2201 e-mail: sasakawa@blue.ocn.ne.jp