- Volume 68 , Number 1
- Page: 1–0
Inhibition of metabolism and growth of Mycobacterium leprae by gamma irradiation
ABSTRACT
Mycobacterium leprae is uncultivable on artificial medium, but viability can be maintained without multiplication for a limited time in vitro. In this study, we evaluated gamma-irradiation (γ-irr) as a means to kill this slowly growing organism. Freshly harvested, viable, athymic, nulnu mouse-derived M. leprae were exposed to varying doses of γ-irr f rom a 60Co source. Two indicators of bacterial viability were determined: metabolism, measured by oxidation of 14C-palmitic acid to 14CO2 in the BACTEC 460 system, and multiplication, measured by titration in the mouse foot pad. γ-Irr of both M. leprae and M. lufu, a cultivable control mycobacterium, resulted in a dose-dependent inhibition of viability. γ-Irr of up to 103 rad had little effect on the metabolic activity of either organism. For M. leprae, 104-105 rad caused an intermediate inhibitory effect; whereas 10'' rad yielded almost total inhibition. In the mouse foot pad assay, up to 106 rad had little effect on M. leprae growth; however, lucrad resulted in at least a 2-log reduction in the number of bacilli recovered and no M. leprae growth was measurable after exposure to 106 rad. With M. lufu, 105 rad inhibited metabolic activity by 99 % and caused >2-log reduction in the number of colony forming units (CFU). No CFU of M. lufu were recovered after exposure to 106 rad. Scanning electron microscopy revealed the presence of some aberrant protrusions on the cell surface of lethally irradiated M. leprae; whereas boiling and autoclaving caused obvious morphological denaturation. These data suggest that γ-irr is an effective way to kill M. leprae witbout causing extensive damage to the cell architecture. Killing M. leprae by γ-irr may be preferable when comparing cellular responses to live versus dead bacilli in vitro and in vivo.RÉSUMÉ
Mycobacterium leprae n'est pas cultivable sur milieu artificiel. Cependant, une certaine viabilité peut être maintenue sans multiplication pendant un temps limité in vitro. Dans cette étude, nous avons évalué l'irradiation aux rayons gammas (γ-irr) comme outil pour inactiver cet organisme à croissance lente. Des M. leprae. récemment récoltées de souris nues athymiques nii/nii. furent exposées à des doses croissantes de γ-irr provenant d'une source de 60Co. Deux indices de viabilité bacillaire furent déterminés: le métabolisme, estimé en mesurant la quantité d'oxydation du 14C présent dans l'acide palmitique en 14CO2 à l'aide du système BACTEC460, et la multiplication, mesurée par la tilration bacillaire des pattes de souris inoculées. γ-Irr a permis d'obtenir une inhibition de viabilité dose-dépendante à la fois de M. leprae et de M. lufu. une mycobactérie contrôle cultivable. Jusqu'à 10' rads, γ-irr n'a eu qu'un effet très limité sur l'activité métabolique des 2 organismes testés. Un effet inhibiteur intermédiaire a été observé pour M. leprae lors d'une exposition à 104-105 rads, tandis que 106 rads a causé une inhibition métabolique presque complète. Le test d'inoculation dans la plante des pieds de la souris a révélé qu'il n'y avait que peu d'effets sur la croissance de M. leprae à des doses inférieures ou égales à 104; cependant, 105 rads ont résulté en une réduction de 2 unités logarythmiques de base 10 du nombre de bacilles récupérés et il n'y avait pas de croissance mesurable de M. leprae après une irradiation de 106 rads. Concernant M. lufu, 105 rads ont provoqué une inhibition à 99% de l'activité métabolique et causé une réduction de 2 unités logarythmiques de base 10 du nombre d'unités capable de former des colonies (CFU). L'exposition à 10" rads a inhibé toute capacité à former des colonies (absence de CFU) chez M. lufu. L'examen au microscope électronique à balayage a révélé la présence de quelques protrusions anormales de la surface bacillaire lorsque M. leprae fut irradiée à des doses léthales, tandis que l'inactivation par la chaleur humide ou l'eau bouillante a provoqué une dénaturation évidente à l'examen morphologique. Ces données suggèrent que γ-irr est une façon aflicace de tuer M. leprae qui évite de provoquer des altérations profondes de l'architecture cellulaire. L'inactivation de M. leprae par γ-irr est probablement préférable d'emploi lorsque l'on veut comparer les reponses cellulaires aux bacillcs vivants ou morts in vitro ou in vivo.RESUMEN
Mycobacterium leprae no es cultivable en medios artificiales pero puede mantenerse viable, sin replicarse, por periodos limitados de tiempo. En este estudio se evaluó el electo de la irradiación gamma (γ-irr) sobre la viabilidad de este microorganismo de lento crecimiento. Se expusieron suspensiones frescas de M. leprae obtenidas de la almohadilla plantar del ratón desnudo al efecto de varias dosis de γ-irr emitida por una fuente de 60Co. Como indicadores de viabilidad de los bacilos se midieron su capacidad para oxidar al l4C-ácido palmítico en el sistema BACTEC 460 y su habilidad para replicarse en la almohadilla plantar del ratón. La irradiación gamma de M. leprae y de M. Iufu, una micobacteria cultivable usada corno control, redujo la viabilidad de ambos microorganismos en un forma que fue dependiente de dosis. La γ-irr con hasta 104 rad tuvo poco efecto sobre la actividad metabólica de los dos microorganismos. Para M. leprae. 104- 105 rad tuvieron un efecto inhibitorio intermedio, mientras que 106 rad causaron una inhibición casi total. En el ensayo de la almohadilla plantar, hasta 106 rad tuvieron poco efecto sobre la viabilidad de M.leprae; sin embargo, 105 rad disminuyeron cuando menos en 2 log el número de bacilos recuperados y no hubo crecimiento medible cuando los bacilos se expusieron a 106 rad. Con M. lufu, 105 rad inhibieron su actividad metabólica en un 99% y causaron una reducción mayor a 2 log en el número de unidades formadoras de colonias (UFC). No hubieron UFC de M. lufa cuando se expusieron a 105 rad. La microscopía electrónica de barrido reveló la presencia de estructuras aberrantes en la superficie de M. leprae sujeto a irradiación letal, mientras que su calentamiento a ebullición y al autoclave causó una obvia desnaturalización morfológica. Estos datos sugieren que la γ-irr es una forma efectiva para matar a M. leprae sin causar daño extenso en su arquitectura celular. Esta forma de matar a Ai. leprae podría ser la preferida cuando se comparan las respuestas celulares hacia bacilos vivos y/o muertos, tanto iu viva como in vitro.Mycobacterium leprae, the causative agent of leprosy, is an obligate intracellular pathogen. Although the leprosy bacillus was discovered by Armaucr Hansen over 125 years ago, it has yet to be cultured in vitro on artificial medium. However, viability of M. leprae can be maintained, without multiplication, for a limited time in axenic medium (7-21) and in cultured macrophages, the primary host cells for the bacilli (8,16, l7).
Enormous strides have been made in leprosy research over the past 30 years as researchers have taken advantage of the large numbers of M. leprae that can be harvested from the tissues of infected armadillos (25) and, to a lesser extent, athymic (nude) mice (2,3) Because of the stigma associated with leprosy in general and an irrational fear of the leprosy bacillus, a Class 2 pathogen, nonviable bacilli are commonly used in research. Various methods have been utilized to kill M. leprae before employing it as a research reagent, including formalin fixation, phenol treatment, or heating at 10()ºC (boiling) or 121ºC (autoclaving). With the curious exception that Shcpard has shown that autoclaving actually improves the immunogenicity of an M. leprae vaccine for mice ('*'), these treatments can cause extensive denaturation and structural damage to the bacilli and, thus, may compromise research goals themselves. Gamma-irradiation (γ-irr) has also been used to kill M. leprae. In purification protocols developed in the 1970s, 2.5 x 106 rad appears to have been arbitrarily chosen as a sterilizing dose to render experimentally infected tissues noninfectious, such as the armadillo spleen and liver for the subsequent preparation of M. leprae vaccines and purified antigens (15. 19, 24, 27)
The purpose of this study was to evaluate the minimum dose of γ-irr required to kill scmipurilied suspensions of M. leprae harvested from the nulnu mouse foot pad. This is a simple enough goal for cultivable microorganisms. But in the case of M. leprae, a less direct approach was required. The labor-intensive and tedious mouse foot pad assay (18) was employed to address the seemingly simple question of whether the bacilli harvested from the foot pads were alive or dead. In addition, the development by Franzblau (5.6) of radiorespiromctric assays to measure M. leprae metabolic activity, and the adaptation of these assays to bacilli cultured in mammalian cells in vitro (12,16), made short-term studies of M. leprae feasible. Both methods were employed in the present study to measure the effects of γ-irr on M. leprae. Parallel studies employing the cultivable, nonpathogenic M. lufu were performed for comparison.
MATERIALS AND METHODS
Mycobacterial cultures
M. leprae are maintained in continuous passage in athymic nulnu mice (Harlan Sprague-Dawley, Inc., Indianapolis, Indiana, U.S.A.) by inoculation of 1 x 108 freshly harvested bacilli into both hind foot pads as previously described (1). At 9 months postinfection, foot-pad tissue was aseptically removed and gently disrupted using a hand-held homogenizer (Wheaton Science Products, Mill ville, New Jersey, U.S.A.). The bacilli were purified by differential centrifugation (6), enumerated (20), and used within 48 hr. Absence of contamination of the M. leprae preparation is ensured by subculture onto blood agar plates, thioglycollate medium, tryptic soy broth, Middlebrook 7H11 medium, and Lowenstcin-Jensen medium. The cultivable, environmental mycobacterium, M. lufu (obtained from Dr. Françoise Portaels, Prince Leopold Institute of Tropical Medicine, Antwerp, Belgium) served as a control. M. lufu was cultured in Middlebrook 7H9 medium supplemented with 10% oleic acidalbumin-dextrose-catalase solution (Becton Dickinson, Cockeysville, Maryland, U.S.A.) plus glycerol (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) plus Tween 80 (Sigma), and used while in mid-log phase growth.
γ-Irradiation
M. leprae and M. lufu were exposed to varying doses (102-106 rad) of γ-irradiation (γ-irr) from a source 60Co source (Shepherd Model 484 irradiatior; J. L. Shepherd and Associates, San Fernando, California, U.S.A.).
Determination of mycobacterial metabolic activity
M. leprae. The BACTEC 460 system, which measures the catabolic conversion of 14C-palmitic acid to 14CO2, was used to determine the effects of γ-irr"on mycobacterial metabolic activity. One x 108 M. leprae in 0.1 ml medium were inoculated into BACTEC 460 vials (5 vials/group). The vials were flushed with a gas mixture consisting of 2.5% O2/10% CO2/balancc N, (Air Liquide America Corp, Houston, Texas, U.S.A.), incubated at 33 C, and read every 3 days for 2 weeks. Because M. leprae does not multiply in vitro, maximum BACTEC readings are reached early in the assay and then begin to decline. Both the peak growth index (GI) and the rate of decline are dependent on the viability of the organisms (6).
M. lufu. M. lufu were inoculated into BACTEC 460 vials (5 vials/group) at a concentration of I x 106 bacilli per vial. Control vials were inoculated with unirradiated M. lufu at 1:1, 1:10 and 1:100 dilutions to represent 0%, 90% and 99% inhibition, respectively. Vials were read daily until the 1:100 dilution gave a GI reading of 30 (10)
Determination of mycobacterial growth
M. leprae. M. leprae were inoculated into both hind foot pads of BALB/c mice at a concentration of 1 x 104 bacilli per each hind foot pad. The number of bacilli were enumerated 6 months postinfection (20). In the titration experiment, control mice were inoculated with 101, 103, or 104 M. leprae into each of both hind foot pads. Experimental mice were inoculated with 104 irradiated bacilli. The number of M. leprae were enumerated at 3, 5, 7 and 12 months postinfection. Experimental groups which yielded counts of M. leprae which were less than those obtained from the 103 and 102 control groups indicated at least a 90% and 99% kill, respectively (14).
M. lufu. Irradiated M. lufu were serially diluted in Hanks balanced salt solution (HBSS) (GIBCO, Grand Island, New York, U.S.A.) supplemented with 0.05% Tween 80 (Sigma) and spread on Middlebrook 7H11 agar plates. Cultures were incubated at 37ºC for 14 days, at which time colony forming units (CFU) were enumerated.
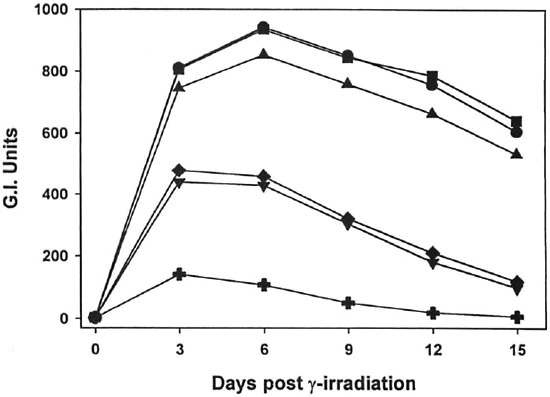
Fig. 1. Effect of γ-irr ou the metabolic activity of M. leprae. Freshly harvested, nu/nu mouse-derived M. leprae were exposed to increasing doses of γ-irr (102 rad,  ;103 rad,
;103 rad,  104 rad,
104 rad,  105 rad,
105 rad,  ; 106 rad, +) from a 60Co source. BACTEC vials were inoculated with l08 bacilli and ineubated at 33ºC. Control vials were inoeulated with unirradiated bacilli (
; 106 rad, +) from a 60Co source. BACTEC vials were inoculated with l08 bacilli and ineubated at 33ºC. Control vials were inoeulated with unirradiated bacilli ( ). Vials were read every 3 days. Data points represent means of quintuplicate samples. Error bars are omitted for clarity, but standard deviations were <10%.
). Vials were read every 3 days. Data points represent means of quintuplicate samples. Error bars are omitted for clarity, but standard deviations were <10%.
Scanning electron microscopy (SEM)
M. leprae were adjusted to l x 109 bacilli/ml and irradiated with 106 rad. Control M. leprae received no irradiation. For morphological comparisons, M. leprae samples were also heated at 100ºC/10 min or were autoclaved at 12lºC/l5 pounds per square inch/15 min. The bacilli in all treatment groups were washed three times in phosphate buffered saline (PBS) and then fixed in 1.25% glutaraldehyde and 2% formaldehyde in 0.1 M sodium cacodylate (CAC) buffer, pH 7.4, for 1 hr at room temperature, washed and resuspended in 0.1 M CAC, and applied to polylysinc-coated glass cover slips. After postlixation in 1% OsO4 pH 7.4, at room temperature for 30 min, they were washed in distilled water dehydrated in ethyl alcohol, critical point dried, mounted with silver paint, sputter coated for 7 min, and viewed on a Cambridge Stereoscan 150 scanning electron microscope.
Statistics
Data were analyzed using a two-tailed parametric Student's t test. All tests were considered significant at p <0.05.
RESULTS
Effects of γ-rr iron mycobacterialmetabolic activity
As shown in Figures 1 and 2, γ-irr of both M. leprae and M. lufu resulted in a dose-dependent inhibition of metabolic activity. γ-Irr of 102 rad had virtually no effect on the oxidation of palmitic acid by either organism [p = 0.7182 (M. leprae), p = 0.9623 (Af. lufu)]. 103 Rad induced a small but significant reduction on M. leprae metabolism (p = 0.0099). However, 104-105 rad caused an intermediate inhibitory effect (p <0.0001) on M. leprae and 106 rad yielded almost total inhibition of metabolic activity (p <0.0001) (Fig. 1). With M. lufu, (Fig. 2) 104 rad inhibited metabolic activity initially but peak Gl readings of 999 were reached by day 8, just one day beyond the unirradiated controls. However, γ-irr of 105 rad and above inhibited metabolic activity of M. lufu by 99% (p <0.0001).
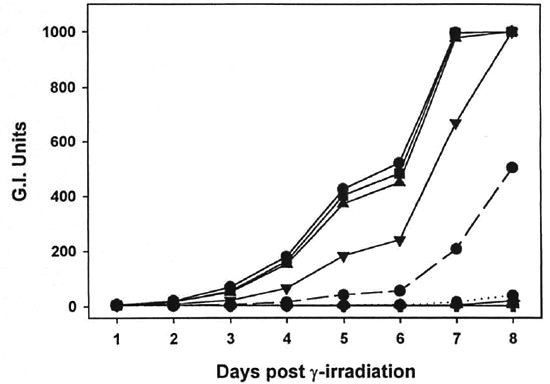
Fig. 2. Effect of γ-irr on the metabolic activity of M. lufu. A log phase culture of M. lufti was exposed to increasing doses (102 rad,  ; 103 rad,
; 103 rad,  ; 105 rad,
; 105 rad,  105 rad,
105 rad,  ; 106 rad, +) of γ-irr from a 60Co source. BACTEC vials were inoculated with 106 bacilli and incubated at 37ºC. Controls vials were inoculated with unirradiated bacilli at 106(
; 106 rad, +) of γ-irr from a 60Co source. BACTEC vials were inoculated with 106 bacilli and incubated at 37ºC. Controls vials were inoculated with unirradiated bacilli at 106( ), 104 (...
), 104 (... ...) and 104 (...
...) and 104 (... ...) organisms to represent 0%, 907c and 99% killing, respectively. Vials were read daily until the 104 inoculum gave a. growth index (Gl) reading of 30. Data points represent means of quintuplícate samples. Error bars are omitted for clarity, but standard deviations were <10%.
...) organisms to represent 0%, 907c and 99% killing, respectively. Vials were read daily until the 104 inoculum gave a. growth index (Gl) reading of 30. Data points represent means of quintuplícate samples. Error bars are omitted for clarity, but standard deviations were <10%.
Effect of γ-irr on mycobacterial growth
The effect of γ-irr on mycobacterial growth was next determined. As shown in Figure 3, the dose of γ-irr was inversely proportional to the number of CFU of M. lufu recovered. γ-Irr of up to 104 rad did not kill the organisms. In contrast, 10' rad caused a 1 log-reduction in the number of CFU of M. lufu and 105 rad reduced CFU by a 3-log reduction. γ-Irr of 106 rad sterilized the culture of M. lufu since no CFU were recovered (limits of detection of assay = 10 bacilli).
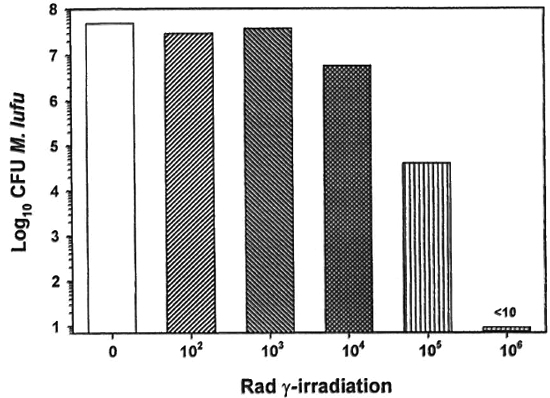
Fig. 3. Effect of γ-irr on the growth of M. Itifu. A log phase culture of M. Itifu was exposed to increasing doses of γ-irr from a 60Co source source. Bacilli were serially diluted and plated on Middlebrook 7HII plates. Control plates were inoculated with unirradiated bacilli. Cultures were incubated at 37 ºC for 14 days and colony forming units units(CPU) were enumerated.
The mouse foot pad assay was used to measure the growth of M. leprae. Multiplication of unirradiated M. leprae peaked at ~106 bacilli per foot pad 6 months postinfection (Fig. 4). Up to 104 rad had little effect on M. leprae growth; however, 105 and 106 rad resulted in no measurable M. leprae growth.
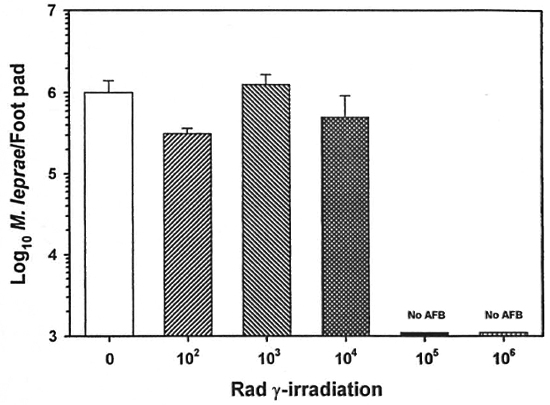
Fig. 4. Effect of γ-irr on the growth of M. leprae. Freshly harvested, nulnu mouse-derived M. leprae were exposed to increasing doses of γ-irr from a 60Co'source. Bacilli were diluted in PBS, and BALB/c mice were inoculated with 5 x 103 M. leprae in each hind foot pad. Control mice were inoculated with unirradiated bacilli. Foot pads were harvested 6 months postinfection and the bacilli enumerated. Results are mean ± SI).
In the mouse foot pad titration experiment (Fig. 5), control mice were inoculated with 104, 103 and 102 M. leprae to represent 0%, 90%, and 99% killing, respectively (Fig. 5). Experimental mice were inoculated with 104 irradiated bacilli. Peak counts of 106 AFB were reached 5 months postinfection in control mice inoculated with 103 and 102 M. leprae peaked later at 7 months postinfection. Experimental mice which had been infected with 104 irradiated M. leprae exhibited a dose response dependent upon the level of γ-irr. Inoculation with bacilli which had been irradiated with 104 rad showed a growth pattern similar to the 103 control, indicating 90% killing of the M. leprae with this does of γ-irr. 105 Rad killed >99% of the organisms since barely countable numbers were detected 5 months postinfection and no AFB were seen at 7 and 12 months postinfection.
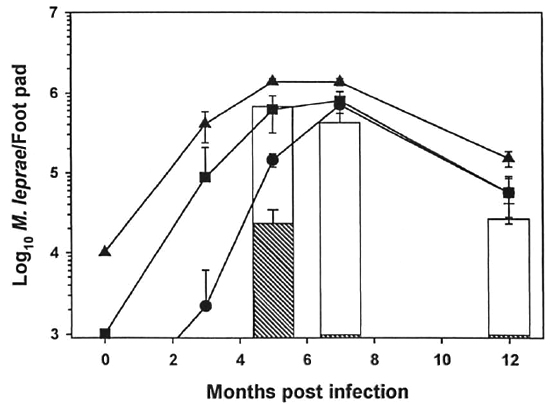
Fig. 5. Titration of γ-irr M. leprae in the mouse foot pad. Freshly harvested, nulnu mouse-derived M. leprae were exposed to 104 rad (open bar) and I(T rad (hatched bar) of γ-irr from a "Co source. Bacilli were diluted in PBS, and BALB/c mice were inoculated with 104 M. leprae in each hind foot pad. Control mice were inoculated with unirradiated M. leprae at 103 ( ), 103 (
), 103 ( ), and 102 (
), and 102 ( ) bacilli per each hind foot pad. Foot pads were harvested 3, 5, 7, and 102 months postinfection. Results are mean ± S.D. (Zero time counts are theoretical).
) bacilli per each hind foot pad. Foot pads were harvested 3, 5, 7, and 102 months postinfection. Results are mean ± S.D. (Zero time counts are theoretical).
S.E.M.
Control and irradiated M. leprae, as well as heat-killed and autoclaved bacilli, were examined by S.E.M. to determine changes in cellular surface morphology (Fig. 6). Control bacilli had a mostly smooth, elongated appearance with some wrinkling and ruffling of the surface. M. leprae which had been exposed to 106 rad also had a mostly smooth appearance, but with some blebbing and protrusions on the cell surface. Heatkilled organisms and autoclaved bacilli both exhibited extensive denaturation and destruction of the cell integrity.
Fig. 6. S.E.M. of M. leprae. Effects of various methods of killing on the morphology of M. leprae. Control bacilli (A) were smooth and elongated, with some wrinkling of the cell surface. γ-Irr M. leprae (106 rad) (B) showed a similar appearance to the control bacilli, but had an increased incidence of small protrusions on the cell surface. Extensive denaturation was observed in the boiled (C) and autoclaved (D) M. leprae preparations.
DISCUSSION
Our results demonstrate that γ-irr is an effective method for killing semipurified suspensions of M. leprae. There is no reason to believe that less purified preparations of M. leprae, i.e., infected tissues, would be more resistant to γ-irr. Viability was determined by measuring bacterial metabolism and growth of the organisms, and both methods established that γ-irr exerts a deleterious effect on M. leprae in a dose-dependent manner. Furthermore, bacterial mortality ensued without extensive structural denaturation to the cell architecture.
Exposure of bacteria to ionizing radiation results in numerous types of DNA lesions, including single- and double-strand breaks, crosslinks, and base modifications (11). The hydroxyl radical is the major reactive species generated in aerated aqueous solutions following γ-irr, and it is highly reactive toward the 5-6 double bond of thymine residues (9). In addition to modifying the pyrimidine bases, hydroxyl radical also forms purine adducts (4 ,13,26). If left unrepaired, these forms of base damage are read incorrectly by the implicative polymerase, resulting in base substitution mutations. It is generally believed that the double-strand break is the most biologically significant of the lesions formed (11), and few prokaryotes have the ability to survive more than a few such lesions. A pair of double-strand breaks can result in the loss of all genetic material lying between the break points. A sufficiently large deletion is likely to remove an essential gene, killing the organism.
Two indicators of bacterial viability were determined. The first, bacterial metabolism, was assayed by inoculation of each species into the BACTEC 460 system. This procedure measures the oxidation of 14C-palmitic acid to 14CO2, γ-Irr of both M. leprae and M. lufu resulted in a dose-dependent inhibition of metabolic activity. Up to 103 rad had little effect on either organism, 104-105 rad yielded intermediate inhibitory effects, and 106 rad essentially inhibited metabolic activity of both organisms entirely. While measurement of palmitic acid oxidation in the BACTEC 460 system is an excellent assessment of mycobacterial viability, growth of the organisms is the ultimate indicator of viability. Therefore, a second method, bacterial multiplication, was evaluated for M. leprae using the mouse foot pad assay and for M. lufit by plating serial dilutions on 7H11 agar plates for enumeration of CFU. Again, a dose response was observed and 10'' rad inhibited growth of both organisms completely.
Of interest is the slight disparity between the comparative effects of 1CP rad on the viability of M. lufu and M. leprae where a 3-log|( ) decrease in CFU was seen in M. lufu compared with the apparent complete loss of viability of M. leprae in the foot pad assay. These findings could represent the relative insensitivity of the in vivo M. leprae assay in comparison to the in vitro M. lufu CFU assay. Alternatively, unlike M. lufu growth on 7H11 agar, intracellular growth in the foot pad tissues is not only a manifestation of M. leprae viability, but also of its pathogenicity and the innate and induced host defenses of the mouse. Even slight damage to the pathogen by γ-irr could tip the survival balance in favor of the host.
Examination of the surface morphology of M. leprae using S.E.M. revealed that γ-irr appeared to be a less damaging method for killing the bacilli than either heating at 100ºC or autoclaving. Morphologically, irradiated organisms exhibited a generally smooth appearance, similar to that observed in unirradiated bacilli, although a blebbing on the cell surface was observed with increased frequency. In contrast, irradiated M. leprae did not exhibit the blatant denaturation observed in the boiled and autoclaved samples.
Therefore, γ-irr at 106 rad is a highly effective means to kill suspensions of M. leprae. These findings arc applicable to leprosy research in several areas. Our own research has focused on the relationship between the leprosy bacillus and its preferred host cell, the macrophage (12), and marked differences between the effects of live and killed bacilli have been reported, especially in the study of phagosomc-lysosome fusion (22) and the effects of live and killed M. leprae on macrophage afferent and efferent effector functions (23). But the harsh methods used to kill the organisms may have unduly affected the bacilli. Because of its complete inhibition of bacterial metabolism and growth, yet mild morphological changes, γ-irr may be the preferred technique when comparing cellular responses to live versus dead bacilli in vitro and in vivo. γ-Irr should also become the preferred method to kill M. leprae that will be shipped internationally under the mandated new, stricter guidelines for transporting infectious agents (IATA Dangerous Goods Regulations 1.3.3.1).
Acknowledgment. We thank J. P. Pasqua and Greg McCormick tor excellent technical assistance.
REFERENCES
1. Adams, L. B., Gillis, T. P., Hwang, D. H. and Krahenbuhl, J. L. Effects of essential fatty acid deficiency on prostaglandin E2 production and cell-mediated immunity in a mouse model of leprosy. Infect. Immun. 65 (1997) 1152-1157.
2. Chehl, S., Ruby, J., Job, C. k. and Hastings, R. C. The growth of Mycobacterium leprae in nude mice. Lepr. Rev. 54 (1983) 283-304.
3. Colston, M. J. and Hilson, G. R. Growth of Mycobacterium leprae and M. marinum in congenitally athymic (nude) mice, nature 262 (1976) 736-741.
4. DlZDAROGLU, M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on "/-irradiation in aqueous solution. Biochemistry 24 (1985) 4476-4481.
5. FRAN/BLAU, S. G. Oxidation of palmitic acid by Mycobacterium leprae in an axenic medium. J. Clin. Microbiol. 26(1988) 18-21.
6. FRANZBLAU, S. G. Drug susceptibility testing of Mycobacterium leprae in the BACTEC 460 system. Antimicrob. Agents Chemother. 33 (1989) 2115-2117.
7. FRAN/BLAU, S. G. and Harris, E. B. Biophysical optima for metabolism of Mycobacterium leprae. J.Clin. Microbiol. 26 (1988) 1124-1129.
8. Fukutomi, Y., McCormick G., Pasqua, J. P., Krahenbuhl, J. L., Toratani, S., Matsuki, G. and Matsuoka, M. Elongation of M. leprae in macrophages cultured in the presence of IL-10. (Abstract) Int. J. Lepr. 66 (1998) 119A.
9. Hariharan, P. V. and Cerutti, P. A. Formation of γ-ray induced thimine damage in Micrococcus radiodurans. J. Mol. Biol. 66 (1972) 65-81.
10. Heifets, L. B., Lindholm-Levy, P. J. and Flory, M. Comparison of bacteriostatic and bactericidal activity or isoniazid and ethionamide against Mycobacterium avium and Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 143 (1991) 268-270.
11. Hutchinson, F. Chemical changes induced in DNA by ionizing radiation. Prog. Nucl. Acid Res. Mol. Biol. 32(1985) 115-154.
12. Krahenbuhl, J. L. and Adams, L. B. The role of the macrophage in resistance to the leprosy bacillus. In: Macrophage-Pathogen Interactions. New York: Marcel Dekker, Inc., 1994, pp. 281-302.
13. Kuchino, Y, Mori, E, Kasai, H., Inoue, H., Iwai, S., Miura, K., Oiitsuka, E. and Nishimura, S. Misreading of DNA templates containing 8-hy droxyguanosine at the modified base and adjacent residues. Nature 327 (1987) 77-79.
14. Levy, L. Death of Mycobacterium leprae in mice and the additional effect of dapsone administration. Proc. Soc. Exp. Bio. Med. 135 (1970) 745-749.
15. Marques, M. A. M., Chitai.e, S., Brennan, P. J. and PESSOLANI, M. C. V. Mapping and identification of the major cell wall-associated components of Mycobacterium leprae. Infect. Immun. 66 (1998)2625-2631.
16. Ramasesh, N., Adams, L. B., Fkanzbi.au , S. G. and Krahenbuhl, J. L Effects of activated macrophages on Mycobacterium leprae. Infect. Immun. 59 (1991) 2864-2869.
17. Ramasesh, N., Hastings, R. C. and Krahenbuhl, J. L. Metabolism of Mycobacterium leprae in macrophages. Infect. Immun. 55 (1987) 1203-1206.
18. Shepard, C. C. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112 (1960) 445-154.
19. Shepard,C. C, Draper, P., Ri is. R. J. and Lowe, C. Effect of purification steps on the immunogenicity of Mycobacterium leprae. Br. J. Exp. Pathol. 61 (1980)376-379.
20. Shepard, C.C. and MacRae,D.H. A method for counting acid-fast bacteria. Int. J. Lepr. 36 (1968) 78-82.
21. SHEPARD, C. C. and McRaE, D. H. Mycobacterium leprae: viability at 0ºC, 31ºC, and during freezing. Int. J. Lepr. 33 (1965) 316-323.
22. Sibley, L. D., Franzbiau , S. G. and Krahenbuhl, J. L. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect. Immun. 55 (1987) 680-685.
23. Sibley, L. D. and Krahenbuhl, J. L. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae. Infect. Immun. 56(1988) 1912-1919.
24. STANFORD, J. L. Skin testing with mycobacterial reagents in leprosy. Tuberc. Lung Dis. 65 (1984) 63-74.
25. Truman, R. and Sanchez, R. Armadillos: models for leprosy. Lab. Anim. 22 (1993) 28-32.
26. van HEMMEN, J. J. and Bi.eichrodt, J. F. The decomposition of adenine by ionizing radiation. Radiat. Res. 46 (1971) 444-456.
27. Weir, R. E., Brennan, P. J., Buti.in, C. R. and DOCKRELL, H. M. Use of a whole blood assay to evaluate in vitro T cell responses to new leprosy skin test antigens in leprosy patients and healthy subjects. Clin. Exp. Immunol. 116 (1999) 263-269.
1. Ph.D., Laboratory Research Branch, GWL.Hansen's Disease Center at Louisiana State University, PO. Box 25072, Baton Rouge, L.A 70894,U.S.A.
2. M.S.; Laboratory Research Branch, GWL.Hansen's Disease Center at Louisiana State University, PO. Box 25072, Baton Rouge, L.A 70894,U.S.A.
3. Ph.D., Laboratory Research Branch, GWL.Hansen's Disease Center at Louisiana State University, PO. Box 25072, Baton Rouge, L.A 70894,U.S.A.
4. Ph.D., Department of Biological Sciences, Louisiana State University, Baton Rouge, LA, U.S.A.
Reprint requests to Dr. Adams at the above address or FAX: 1-504-346-5786; e-mail: ladamsl@lsu.edu
Received for publication on 2 August 1999.
Accepted for publication on 23 September 1999.