- Volume 68 , Number 1
- Page: 40–8
Interferon-gamma responses to candidate leprosy skin-test reagents detect exposure to leprosy in an endemic population
ABSTRACT
New tools for the detection of leprosy exposure in a community will be necessary for the eradication of leprosy. Candidate leprosy skin-test antigens derived f rom the fractionation of the leprosy bacillus into cytoplasmic and cell-wall proteins free of immuno-inhibitory mycobacterial lipoglycans and carbohydrates were used in an overnight blood test to determine whether exposure to leprosy can be detected by the production of the cytokine interferon gamma ( IFN-γ ). Strong IFN-γ responses were detected in leprosy contacts to both skin-test antigens compared with control subjects f rom the same endemic communities. There was little response in patients with tuberculosis. Responses were greatest in contacts with recent leprosy exposure. The implications of these findings for the application of these reagents in a field trial as skin tests to detect exposure to leprosy are discussed in light of the strong association between overnight IFN-γ to PPD and the tuberculin skin-test responses previously reported.RÉSUMÉ
De nouvaux outils pour la détection de l'exposition au bacille de la lèpre dans les communautés vont être nécessaires pour pouvoir éradiquer la lèpre. Des antigènes, candidats pour un futur test cutané dépistant l'infection, furent obtenus à partir du fractionnement du bacille de la lèpre en protéines intracytoplasmiques et en protéines provenant de la paroi cellulare, dépourvues de lipoglycanes et d'osés immuno-inhibiteurs. Ces préparations furent testées à l'aide d'un essai sanguin rapide (résultats le jour suivant) qui permet de détecter l'infection par le bacille de la lèpre par la production de la cytokine interferon gamma ( IFN-γ ). De robustes productions d' IFN-γ furent détectées parmi les contacts de patients lépreux en réponse aux deux antigènes candidats pour des tests cutanés, lorsque comparé à des sujets contrôles provenant des mêmes communautés endémiques. Il n'y eut qu'une faible réponse chez les patients souffrant de tuberculose. La réponse fut la plus élevée parmi les contacts ayant eu une exposition récente à la lèpre. Les conséquences de ces résultats pour l'application de ces réactifs dans des essais cliniques sur le terrain comme tests cutanés pour détecter l'exposition au bacille de la lèpre sont discuttées, à la lumière de la forte association récemment rapportée entre le test rapide à l' IFN-γ en réponse au test cutané au PPD et à la tuberculine.RESUMEN
Para erradicar la lepra se requiere de nuevas herramientas capaces de detectar la exposición a la enfermedad en una comunidad. En este estudio se probaron dos derivados micobacterianos que podrían ser útiles como antígenos en pruebas intradérmicas y que incluyen componentes citoplásmicos y de pared del bacilo de la lepra, libres de lipoglicanas y carbohidratos inhibidores. Estos antígenos se usaron en una prueba sanguínea para determinar si la exposición a la lepra se puede detectar por la producción de interferon gamma (IFNy). Se detectaron fuertes respuestas de IFNy contra ambos antígenos en los contactos de los pacientes con lepra en comparación con las respuestas de sujetos control de las mismas comunidades endémicas. Hubo poca respuesta en los pacientes con tuberculosis. Las respuestas fueron mayores en los contactos con exposición reciente a la lepra. La posibilidad de utilizar estos reactivos en pruebas de campo como antígenos en pruebas intradérmicas tendientes a detectar la exposición a la lepra se discute sobre la base de la fuerte asociación entre la producción de IFN-γ en respuesta al PPD y los resultados de las pruebas dérmicas a la tuberculina reportadas previamente.Infection with Mycobacterium leprae precedes the development of clinical leprosy by many months or years (14) This subclinical state is resolved in the majority of people by an effective natural immunity, the compound of exposure to environmental mycobacteria and BCG vaccination. Studies in Ethiopia show that on exposure to leprosy there is an engagement with the adaptive immune system (7) which causes the expansion of clones of leprosγ-specific T cells which produce cytokines such as interferon-gamma ( IFN-γ ).
Leprosy control measures have to date focused on the delivery of an effective therapy to those with clinical leprosy. Implicit in this strategy is that as the number of infectious cases are cured the transmission of the leprosy bacteria in the community will be disrupted. There is, however, no measure of exposure to leprosy applicable to community studies. Such a measure is needed to measure the impact of multidrug therapy (MDT) on transmission and to estimate the number of new cases arising in different communities.
Over the past years, work at Colorado State University, Fort Collins, Colorado, U.S.A., has focused on the development of fractions of the leprosy bacillus, free of immuno-inhibitory lipids, which would be suitable for skin tests (9,12) Studies in Nepali leprosy patients have shown that these antigens are strong inducers of cell proliferation and IFN-γ production in patients with leprosy (16,20)
In the present work we have used a simple whole blood overnight culture to measure the production of IFN-γ in response to these skin-test antigens. The production of IFN-γ in response to purified protein derivative (PPD) of M. tuberculosis in a similar overnight blood culture has been shown to correlate strongly with skin-test reactivity to PPD (18). We have examined the responses of leprosy contacts and Nepali control subjects and patients with tuberculoid leprosy, lepromatous leprosy and tuberculosis to assess the ability of the antigens to induce IFN-γ and, thus, the potential of the antigens to induce skin-test reactions. We also sought to examine the utility of these reagents in detecting exposure to leprosy by comparing the responses in known contacts with those in the same communities without known exposure.
MATERIALS AND METHODS
Antigens. M. leprae sonicate (MLS), M. leprae cytosolic fraction (MLSA-LAM) and cell-wall fraction (MLCwA) were prepared at the University of Colorado under a National Institutes of Health (NIH) contract NO 1-A 1-5562. Tuberculin PPD (Batch RT47) was supplied by Staten Sermminstitut, Copenhagen, Denmark. Phytohemagglutinin (PHA) was supplied by Sigma-Aldrich Co., Poole, U.K.
Study population. These studies were approved on ethical and technical grounds by the Nepal Health Research Council of His Majesty's Government, Nepal. A total of 258 subjects were tested. Healthy unexposed individuals (N = 43) without signs of clinical leprosy and without a history of leprosy in their families or any known contact with leprosy were recruited from the local university. Leprosy patients being treated with MDT were defined on clinical grounds as tuberculoid (TT/BT) leprosy patients (N = 22) and lepromatous (BL/LL) leprosy patients (N -= 23). Tuberculosis (TB) patients (N = 20) were patients with pulmonary TB diagnosed on clinical grounds who were receiving anti-TB drug therapy.
Healthy leprosy contacts (N = 150) were household and nonhouschold contacts of currently active or inactive leprosy cases. A household contact was defined as a person, usually a relative, currently living in the same house as a leprosy patient; a nonhouschold contact was defined as a person, usually a nonrelative, living in the same village, or someone who visited the patient frequently. Data on the index case (leprosy type, smear positivity, treatment type and length) and the contact's exposure (length of total exposure, length of exposure to untreated disease, and relationship to the index case) were also recorded.
Blood samples. Blood samples were drawn after obtaining informed consent from each study participant. Samples were collected into 20-ml sterile tubes containing preservative-free heparin (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) at 10 U/ml. The samples were incubated with antigens within 2 to 4 hr of collection.
Overnight whole blood assay. One ml aliquots of undiluted whole blood were incubated in wells of a 24-well, tissue culture plate. Antigens (MLSA-LAM, MLCwA, M. leprae sonicate, PPD) and mitogen PHA in equal volumes of RPMI media (Sigma) were added to each well to give a final concentration of 10 µg/ml. After a 24-hr incubation at 37ºC in 5% CO2 plasma was harvested from the top of the blood cultures. Approximately 300 ul of plasma was harvested per ml of blood and frozen at -20ºC for subsequent IFN-γ measurement.
IFN-γ measurements. IFN-γ was measured by an enzyme-linked immunosorbant assay (ELISA) using paired monoclonal murine antibodies according to the manufacturer's instructions (Pharmingcn, San Diego, California, U.S.A.). ELISA plates (Immulon 2; Dynatcch, Chantilly, Virginia, U.S.A.) were coated with a mouse antihuman IFN-γ monoclonal antibody (2 µg/ml) in carbonate buffer, pH 9.6, overnight at 4ºC. The wells were then blocked with 200 ul 3% bovine serum albumin (BSA) in phosphate buffered saline (PBS) per well for 2 hr at room temperature (RT); 50 µl of each culture supernatant was added to the wells in duplicate. Serial dilutions of recombinant human IFN-γ (Lot 321.01012; Biogcn SA, Geneva, Switzerland) diluted in RPMI with 5% heat-inactivated pooled human serum were added to the control wells in a range from 1 to 250 U/ml, and the plates incubated at 4ºC overnight. Then the plates were washed four times with PBS containing 0.05% Tween-20 (PBST), and biotin-labeled antihuman IFN-γ (1 µg/ml, 100 µl per well) was added. The plates were incubated for 45 min at RT and then washed six times. Avidin peroxidase (1 mg/ml) diluted 1 in 800 in \ % BSA/PBST was added to the plates and they were incubated for 30 min at RT. Color reaction was developed by adding 100 µl of 0.4 mg/ml o-phenylenediaminc (Sigma) in citrate phosphate buffer (pH 5) containing 0.006% hydrogen peroxide. The reaction was stopped with an equal volume of 2.5 N sulfuric acid, and the plates were read at a wavelength of 492 nm using a Dynatech MRX Plate Reader. IFN-γ results were expressed as mean units of IFN-γ (U/ml) of duplicate wells after subtraction of any nonspecific IFN-γ production in nonstimulatcd cultures. The detection limit of the IFN-γ ELISA was 1 U/ml.
T-cell subset depletion. CD4+ or CD8+ T cells were depleted from whole blood using Dynabeads M450-CD4/CD8 magnetic beads according to the manufacturer's instructions. Cells depleted of CD4+ orCD8+ T cells were incubated overnight with 10 µg/ml of MLSA-LAM as described above and IFN-γ was measured. Twenty depletion experiments were performed (4 controls, 4 healthy leprosy contacts, 4 TT/BT patients, 4 BL/LL patients and 4 TB patients).
Statistics. All statistical tests were performed using the "Stata" (Version 5) statistical program. The variance of the log transformed data was compared among groups and found to be similar in distribution, except for the PPD results in TB patients compared with control groups. The IFN-γ responses in the study groups were compared by the F test to assess the significance of the differences in the means between groups.
The confounding effects of age, sex, type of contact, index case smear status and length of total exposure (but not length of exposure to untreated disease) was controlled for by the use of analysis of covariance (ANCOVA) and adjusted means were calculated. Correlation between continuous variables was measured by the Spearman rank correlation coefficient using raw data.
RESULTS
A total of 258 subjects were tested in the overnight assay. Of these, 248 gave measurable IFN-γ of greater than or equal to 1 U/ml above that in control wells without antigen. Ten contacts who did not respond above background to any antigen were excluded.
IFN-γ responses in the different groups are shown in Table 1. The leprosy contact group had the strongest IFN-γ responses to the two candidate skin-test reagents, significantly greater than those of controls. Responses of the tuberculoid leprosy patients to MLSA-LAM were also greater than controls. Lepromatous leprosy patients made significantly less IFN-γ than controls to MLCwA and MLS. Responses to the leprosy skin-test antigens in TB patients were not significantly greater than controls. The individual responses in the different study groups to the skin-test reagents MLSA-LAM and MLCwA are shown in The Figure.
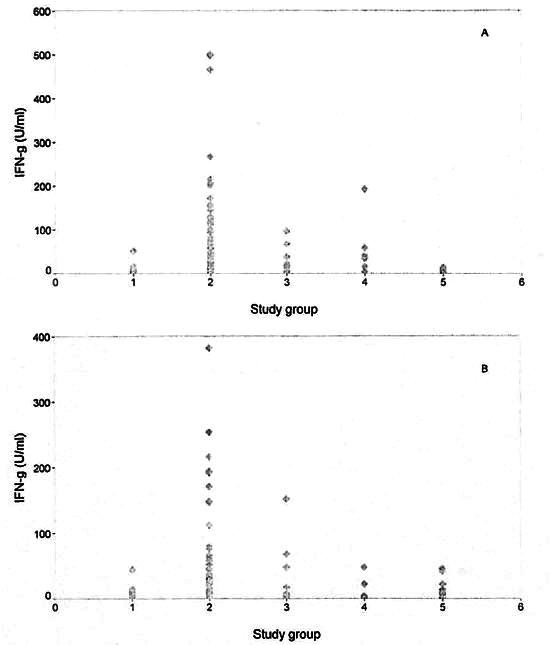
The figure. Interferon-gamma ( IFN-γ ) responses in study groups to new leprosy skin-test antigens. A = MLSA-LAM; B = MLCwA. 1 = Endemic controls (N = 43); 2 = healthy leprosy contacts (N = 140); 3 = TT/BT leprosy patients (N = 19); 4 = BL/LL leprosy patients (N = 23); 5 = tuberculosis patients (N = 20).
The 140 contacts remaining in the study after nonrcspondcrs were excluded were 75 males and 65 females with an age range of 9 to 78 years (average 30.4 years); 112 were household contacts of leprosy patients and 28 were nonhousehold contacts (as described above). The total length of exposure recorded for 115 of the patients ranged from 1 month to 44 years (average 75 months). Similarly, data available on 113 of the contacts showed that the period of exposure to an untreated patient ranged from 1 month to 28 years (average 29.7 months). This was based on the patient's estimate of the length of time between the appearance of the first symptoms of clinical leprosy and the beginning of antileprosy drug treatment.
The index cases of the contacts were 117 multibacillary (MB) and 21 paucibacillary (PB) cases (defined by treatment category) and unknown in 2 cases. Of the 117 index cases with slit-skin-smear data available, 65 had been skin-smear positive and 52 had been skin-smear negative. All but one of the index cases were treated with MDT. Of the index cases currently under treatment at the time of testing contacts, 37 patients had been treated for less than 1 year and 13 for more than 1 year.
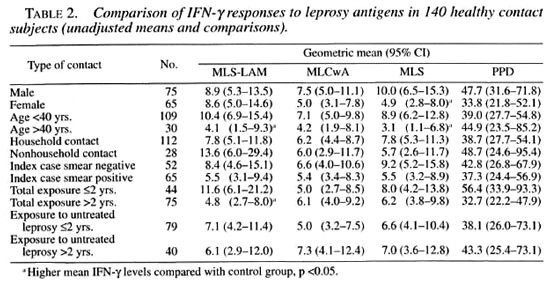
We examined the IFN-γ responses in the subgroups of the 140 leprosy contacts (Table 2). Female contacts had a significantly lower mean IFN-γ response to MLS than did male contacts. Contacts aged less than 40 years had significantly higher IFN-γ responses to MLSA-LAM and MLS than contacts aged more than 40 years.Contacts with reccnt exposure (within thelast 2 years) had signiticantly higher responscs to MLSA-LAM than did contactswith long-tcrm exposurc (more than 2years). There wcre nonsignificant differences between contacts of smear-negative patients and those who had contact with smear-positive patients. The length of exposure to untreated patients appeared to have had no effect on the IFN-γ levels of contacts; this was possibly because exposure to untreated patients had occurred some years previously.
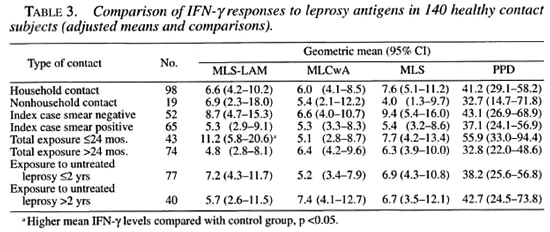
Since there was evidence of age and sex confounding, multiple linear regression was used to derive adjusted means for the contact subgroups (Table 3). When this adjustment was made, only recent exposure was shown to significantly affect the contacts' IFN-γ responses.
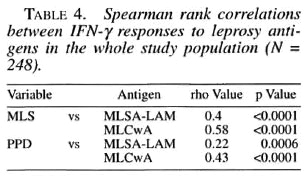
The IFN-γ responses to skin-test antigens and the IFN-γ responses to MLS and PPD were variably but significantly correlated for the whole study group (Table 4). When individual responses were correlated with age or length of exposure, weak, statistically nonsignificant correlations were noted (data not shown).
Depletion of peripheral blood mononuclear cells (PBMC) with magnetic beads coated with monoclonal antibodies revealed that CD4+ cells are the major but not exclusive contributor to 24-hr IFN-γ production. There was no significant difference in the cell subset IFN-γ production between study groups. The combined group median IFN-γ production from undeplcted PBMC was 8.5 U (range 2-230). After depletion of CD4+ cells, the median IFN-γ production was 4.0 U/ml (range 0-73; p = 0.0057) compared with the undepleted IFN-γ level. After depletion with anti-CD84-, the median IFN-γ proauction was 6.0 U/ml (range 0-220; p = 0.07) compared with the undcpleted IFN-γ level.
DISCUSSION
The source of cytokines in short-term blood cultures are "armed effector" T cells (10). These circulating, antigen-specific, memory T cells are able to liberate activating cytokines rapidly on antigen presentation. IFN-γ is the key cytokine in cell-mediated immune responses which control intracellular infections such as leprosy. IFN-γ is also important in the dclaycd-typc hypersensitivity (DTH) reaction, which is the basis of the tuberculin skin test (TST) (4) Recently, a strong correlation has been established between IFN-γ produced in a 24-hr whole blood assay and the tuberculin skin test (,8). The overall specificity of IFN-γ to PPD from M. tuberculosis compared with the TST was 98%, with a sensitivity of 90% in healthy Australian individuals. Thus, a link has been established between the in vitro production of IFN-γ and the DTH response in vivo. This suggests that the leprosy antigens studied here have the potential to induce DTH skin reactions in leprosy contacts. In an earlier study at this center, IFN-γ produced to the MLSA-LAM antigen in a 6-day culture was strongly associated with lepromin skin-test positivity (20).
The IFN-γ responses described here discriminate well between leprosy contacts and endemic controls. This indicates that the preparations are sufficiently leprosy specific so that exposure to other mycobacterial disease, environmental mycobacteria or BCG vaccination does not prime individuals to respond to the skin-test reagents. The leprosγ-specific nature of the skin-test antigens is supported by the observation that the TB patient group also did not make significant IFN-γ responses to the leprosy reagents. Further, the lepromatous leprosy patient group shows significantly reduced IFN-γ responses compared to the control group; this agrees with previous observations of a "hyporesponsive" or non- IFN-γ response to leprosy antigens in lepromatous patients (3).
MLS is the parent material from which MLSA-LAM is derived, and it is similar to the material used in previous skin-test studies. Skin-test surveys in leprosγ-endemic populations using Recs and Convit antigens have shown evidence of nonspecific sensitization in endemic populations. The preparations were not sensitive enough to detect leprosy nor specific enough to confirm a clinical diagnosis of leprosy (8). In the present study, IFN-γ responses to MLSA-LAM and MLCwA (The Figure) show a distinctly stronger response in leprosy contacts compared to nonexposed endemic control subjects and patients with TB.
The finding that contacts recently exposed to leprosy patients had substantially stronger responses to MLSA-LAM is of interest. This difference persisted after adjusting for age, sex and type of contact and smear positivity. One explanation could be that chronic exposure leads to a maturation of the immune response, which downregulatcs IFN-γ production. Alternately, exposure to leprosy may be a time-limited event, which stimulates a population of T cells to produce IFN-γ but, without ongoing stimulation, this memory T-ccll population declines in number or goes into senescence.
Current strategies to measure exposure to leprosy use serology or polymerase chain reaction (PCR). Studies of contacts in The Philippines indicate a greatly increased risk of developing disease among contacts who are seropositive for antibodies to the phenolic glycolipid of M. leprae; however, only 5.9% of all contacts were seropositive (6). Indeed, it now appears from several studies that seropositivity in the majority of contacts may wane without the development of disease (1). Serological responses are measurable only in frank diseases and where the bacillary load is evidenced by a positive skin smear (13). Antibody measurements are less useful in measuring exposure to leprosy in the community since the primary engagement of host immunity in leprosy is the cellular immune response. However, the combined testing of cell-mediated immunity by a leprosy skin test and humoral immunity by antibody measurements may be useful in the diagnosis of the disease.
PCR has revealed the apparent presence of M. leprae DNA in the noses of unaffected people in leprosγ-endemic communities (11). This has aroused much interest in the possibility that M. leprae may be spread by unaffected carriers who may not be close contacts of leprosy patients. A large longitudinal study in Ethiopia and India is now attempting to establish the importance of these findings (5), However, mass screenings of nasal swabs by PCR is an expensive and labor-intensive process; the findings are of yet uncertain epidemiological significance and the methodology is unlikely to be affordable to leprosy programs in the postelimination era.
Despite the lack of specificity of tuberculin and the confounding effect of mass BCG vaccination, national tuberculin surveys are still of use for tuberculosis control ( 2,13 ). Stylbo, et al. (19) have used the TST and tuberculin surveys to assess the decline in the annual risk of infection with tuberculosis, which allow TB control programs to monitor the effect of their interventions on TB incidence. A specific leprosy skin test would allow similar predictions to be derived for leprosy control programs. The important question of whether MDT is disrupting transmission, as well as other questions about leprosy epidemiology, could be answered by community skin-test surveys which measure changes in leprosy exposure.
The reagents described in this report will be tested as skin tests in the first field trial in Nepal within the next 12 months. Safety trials in the U.S.A. (Brennan, unpublished observations) have shown that 9 of 10 nonexposed subjects who received up to 25 µg of the reagents in an intradermal injection did not show any induration. Studies in Nepal will focus on the safety and immunogenicity in an endemic population, and will attempt to establish the specificity of the reagents as skin tests for tuberculoid leprosy.
Further studies will then be required in populations with different leprosy prevalences to measure the strength of the association of skin-test reactivity with leprosy exposure. Later still, longitudinal studies will be required to assess the relative risk of developing leprosy among positive and negative respondéis (l7). Should the in vitro IFN-γ results correlate with in vivo skin-test results in these studies, we will also have established an assay by which to assess other leprosy antigens for their potential use as leprosy skin tests.
Acknowledgment. This work was financially supported by The Leprosy Mission International. The authors would like to thank the patients of Anandaban Leprosy Hospital and their families for their participation in this study. We would also like to thank the students from the Central Department of Microbiology, Tribhuvan University, Kathmandu, Nepal, who donated blood for the control group. A special word of thanks to Mr. K. D. Neupane who coordinated the blood collection from patient contacts.
REFERENCES
1. BAUMGART, K. W., BRITTON, W. J., MULUNS, R. J., BASTEN, A. and BARNETSON, R. ST.C. Subclinical infection with Mycobacterium leprae-a problem for leprosy control strategies. Trans. R. Soc. Trop. Med. Hyg. 87(1993)412-415.
2. BOSMANN, M. C. J., SWAI, O. B., KWAMANGA, D. O., AGWANDA, R., IDUKTTTA, G. and MISUENOVIC, O. National tuberculin survey of Kenya 1986-1990. Int. J. Tuberc. Lung Dis. 2 (1998) 272-280.
3. BRITTON, W. J. Immunology of leprosy. Trans. R. Soc. Trop. Med. Hyg. 87 (1993) 508-514.
4. CHU, C. Q., FIELD, M., ANDREW, E., HASKARD, D., FELDMANN, M. and MAINI, R. N. Detection of cytokines at the site of tuberculin-induced delayedtype hypersensitivity in man. Clin. Exp. Immunol. 90(1992)522-529.
5. CREE, I. A. and SMITH, W. C. Leprosy transmission and mucosal immunity: towards eradication? Lepr. Rev. 69(1998) 112-121.
6. CUNANAN, A., CHAN, G. P. and DOUGLAS, J. T. Risk of development of leprosy among Culion contacts. (Abstract) 15th International Leprosy Congress, Beijing, China, 1998. Int. J. Lepr. 66 (1998) 78A.
7. GODAL, T, LOFGREN, M. and NEGASSI, K. Immune response to M. leprae of healthy leprosy contacts. Int. J. Lepr. 40 (1972) 243-249.
8. GUITE, M. D., ANANTHARAMAN, D. S., NAGARAJU, B., KANNAN, B. and VALLISHAYEE, R. S. Experiences with Mycobacterium leprae soluble antigens in a leprosy endemic population. Lepr. Rev. 61 (1990)132-144.
9. HUNTER, S. W., MCNEIL, M., MODLIN, R. L., MEHRA, V., BLOOM, B. R. and BRENNAN, P. J. Isolation and characterization of the highly immunogenic cell-wall-associated protein of Mycobacterium leprae. J. Immunol. 142 (1989) 2864-2872.
10. JANEWAY, C. A. and TRAVERS, P. Immunobiology: The Immune System in Health & Disease. 2nd edn. Edinburgh: Churchill Livingstone, 1996, pp. 9:40-9:42.
11. KI.ATSER, P. R., VAN BEERS, S., MADJID, B., DAY, R. and Di; WIT, M. Y. L. Detection of Mycobacterium leprae nasal carriers in populations for which leprosy is endemic. J. Clin. Microbiol. 31 (1993)2947-2951.
12. MEHRA, V., BLOOM, B. R., TORIGAN, V. K., MANDICH, D., REICHEL, M., YOUNG, S. M. M., SALGAME, P., CONVIT, J., MCNEIL, M., BRENNAN, P. J. and REA, T. H. Characterization of M. leprae cell-wall associated proteins with the use of T-lymphocyte clones. J. Immunol. 142 (1989) 2873-2878.
13. MENZIES, D. Tuberculin surveys-why? Int. J. Tuberc. Lung Dis. 2 (1998) 263-264.
14. NOORDEEN, S. K. The epidemiology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill-Livingstone, 1985, pp. 15-30.
15. ROCHE, P. W., BRITTON, W. J., FAII.HUS, S. S., WILLIAMS, D., PRADHAN, H. M. and THEUVENET, W. J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. 58 (1990) 480-490.
16. ROCHE, P. W., NEUPANE, K. D. and BRITTON, W. J. Cellular immune response to cell walls of Mycobacterium leprae in leprosy patients and healthy subjects exposed to leprosy. Clin. Exp. Immunol. 89(1992) 110-114.
17. SAMPAIO, E. P., MOREIRA, A. L., KAPLAN, G., ALVIM, M. F. S., DUPPRE, N. C, MIRANDA, C. F. and SAKNO, E. N. Mycobacterium leprae- induced inlerferon-γ production by household contacts of leprosy patients: association with the development 20. of active disease. J. Infect. Dis. 164 (1991) 990-993.
18. STREETON, J. A., DESEM, N. and JONES, S. L. Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. Int. J. Tuberc. Lung Dis. 2 (1998) 443-450.
19. STYI.BO, K., MEIJER, J. and SUTHERLAND, I. The transmission of the tubercle bacilli: its trend in a human population. Bull. Int. Union Tuberc. 42 (1969) 5-105.
20. WEIR, R. E., BRENNAN, P. J., BUTLIN, C. R. and DOCKRELL, H. M. Use of whole blood assay to evaluate in vivo T-cell responses to new leprosy skin test antigens in leprosy patients and healthy subjects. Clin. Exp. Immunol. 116 (1998) 263-268.
1. B.Sc. (Hons); Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
2. M.D.; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
3. M.B.B.S.; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
4. Ph.D., Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
5. Ph.D., Department of Microbiology, Colorado State University, Fort Collins, Colorado, U.S.A.
Reprint requests to Dr. Roche at the above address or FAX 977-1-290-538; e-mail: roche@umn.mos.com.np
Received for publication on 8 July 1999.
Accepted for publication in revised form on 4 November 1999.
This work was presented in part as an oral presentation at the 15th International Leprosy Congress, Beijing, China, 7-12 September 1998 (Abstract IM07).