- Volume 68 , Number 1
- Page: 71–3
Phagocytic myeloperoxidase in leprosy pathogenesis
To the Editor:
It is well known tha t a causative agent of leprosy, Mycobacterium leprae, survives and multiplies in cells of the mononuclear phagocyte line (MPL). MPL cells act as initiators and regulators of the immune response and they are involved in nonspecific resistance and antimicrobicidal activities. In a view of improvement and development of new methods for the prevention and therapy of leprosy, a detailed study of the mechanisms of M. leprae persistence in MPL cells seems to be urgent.
Today a lot of researchers devote themselves to studying myeloperoxidase (MP) of phagocyting cells, especially in chronic infectious and granulomatous diseases, since it is known that the acquired or hereditary deficit of MP activity might favor the development of chronic infectious diseases, infectious complications of chronic infectious diseases or granulomatoses.
According to present-day conceptions, acid hydrolases destroy only those bacteria in phagolysosomes which have been killed by the MP system, nonenzymatic cationic proteins, lysozymc and lactoferrin (9).
Decreased MP activity of neutrophils of peripheral blood has been observed in patients with chronic myelolcukosis and subleukemic myelosis. In this group there was a high percent of the cases with infectious complications (2). With strongly insufficient activity of MP, MPL cells are not capable of killing Candida , staphylococci and various gram-negative microorganisms (10). Low indices of MP activity of peripheral blood phagocytes are found in patients with infiltrated dermatophytoses of the scalp (3) and patients with chronic tonsillitis (1). The data obtained suggest that the level of MP activity of MPL cells might be a reliable criterion for the assessment of their phagocytic ability.
In M. feprae-infected armadillos M. leprae were absent in macrophages with high activity of MP, and, contrary, macrophages with low MP activity contained numerous M. leprae in their cytoplasm (8).
We carried out electron cytochemical investigations of skin granulomas from lepromatous leprosy patients and found that macrophage cytoplasm with low MP activity contained clusters of intact M. leprae and, contrary, intensive lysis of M. leprae was observed in macrophages with high MP activity (5). Our long-term (over 14 years) observations on the possible relationship between the terms of appearance and stability of clinical improvement and activity of phagocytic MP could establish a strong association between the rate of MP activity in macrophages of skin lepromas (as observed at the patient's admittance) and the time of appearance of the first signs of clinical improvement and stability of regress. A high level of MP activity in granuloma macrophages strongly correlated with the fast elimination of M. leprae and the highest peak of effectiveness of therapy administered. Low activity of macrophage MP was correlated with the slow regress of the disease and risk of relapse (6).
Further, the goal was sought to find a correlation between the intensity of phagocytic responses and MP activity in experiments on mice subject to an induced decrease in MP activity in MPL cells, as well as to elucidate whether it is possible to simulate a process of persistence of pathogenic mycobacteria (M. leprae and M. tuberculosis) in MPL cells under the above conditions.
With this aim in mind, the following tasks should be solved: to evolve a method of decreasing the activity of phagocytic MP and to define optimal agent concentration that would not affect cell ultrastructure.
It is well known that one of the main functions of MPL is to bind hydrogen peroxide preventing its cellular accumulation; the enzyme therewith loses its activity. All things considered, we attempted to reduce MP activity in MPL cells by saturating them with hydrogen peroxide solution.
In order to obtain peritoneal macrophages (PM), mice were stimulated by means of intraperitoneal (i.p.) injection of 2% peptone casein solution. Two hr after stimulation and 1-2 hr before M. leprae inoculation the mice were injected i.p. with test solutions of hydrogen peroxide (0.3%, 0.6% and 1.0%) at a dose of 2 ml. Mice were inoculated i.p. with 1 x 106 organisms (M. tuberculosis obtained from patients with pulmonary tuberculosis and M. leprae passed on mice were suspended in 1 ml of salt solution). The mice were sacrificed at 2 hr, 1, 2, 3, 4, 5, and 6 days after inoculation. PM activity was assayed electron microscopically and cytochcmically as well as in an absorption test.
The experimental data showed that in mice injected i.p. with the experimentally chosen 0.6% concentration of hydrogen peroxide at a dose of 2 ml, MP activity in MPL cells was significantly decreased within 6 days, with a simultaneous reduction in phagocytic activity. Electron microscopy of H2G2-treated PM at 6 days showed numerous mycobacterial cells with intact ultrastructure (Fig. la). In control samples, mycobacteria were completely lyscd in 2-3 days (Fig. lb). Initial indices of PM absorption capability (phagocytic index and phagocytic count) markedly increased, but 3 days afterward they leveled off and approached control indices.
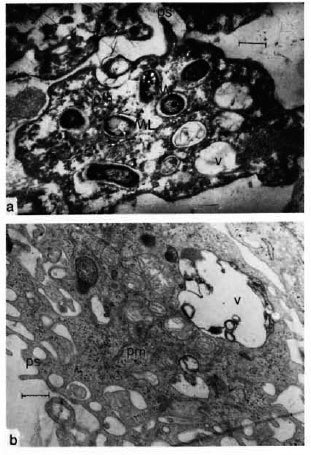
Fig. 1. Electron micrographs of peritoneal macrophages (PM) of test (a) and control (b) mice 6 days after M. leprae (ML) inoculation; ps = pseudopodia, v = vacuoles (uranil acetate and lead citrate stain; bar = 0.5 )µm).
A study of the ultrathin structure of PM showed that a drop in activity of MP 2-3 days after the mice were inoculated was accompanied by a significant decrease in the number of PM pseudopodia and their flattening (macrophages became round) as well as disturbed lysosome-phagosome fusion (4).
Thus, a single saturation of MPL cells with a 0.6% solution of hydrogen peroxide causes reduced MP activity in phagocytes; hence, resulting in long-term persistence of pathogenic organisms (M. tuberculosis and M. leprae) in their cytoplasm.
Based on the above investigations and considering the actuality of developing an optimal leprosy model and identification of factors favoring mycobacterial persistence in host cells, we tried to improve Shepard's technique by selectively acting on the MP system of phagocytes. For this purpose, 1-2 hr before M. leprae inoculation the mice were given a 0.6% freshly prepared solution of hydrogen peroxide at a dose of 0.3-0.5 ml per foot pad. A single injection of hydrogen peroxide resulted in a more intensive multiplication of leprosy bacilli in the mouse foot pads as compared with standard methods of inoculation (Table 1) and a generalization of the infection (M. leprae were seen in print smears of lung and spleen tissue of mice) (Table 2). Electron cytochemistry demonstrated low activity of MP in phagocytic cells, suggesting an important role of the MP system of phagocytes in the mycobacterial killing and pathogenesis of leprosy (7).
Our studies provide an opportunity for a directed alteration of phagocytic activity of MPL cells. A proposed approach to induce phagocytic insufficiency is similar to the mechanism of human immunodeficiencies in a number of chronic infectious and inflammatory diseases, offering strong possibilities for searches and screening immunocorrective agents of selective effect on bactericidal phagocytic system to compensate for MPL insufficiency.
Alexander K. Maslov, M.D.
Leprosy Research Institute
Ostrovsky pas.3
Astrakan 414057, Russia
REFERENCES
1. DRAGOMIRETSKY, V. D. and BAZHORA, Y. I. Assessment of bactericidal system of polymorphonuclear leucocytes in patients with chronic tonsillitis. Laboratornoye Delo. 11 (1986) 649-652.
2. GUSEVA, S. A., TlSHCHENKO, L. M. and GAIDUKOVA, S. N. Myeloperoxidase activity and infectious complications: a relationship in myeloproliferative disorders. Archiv Patologii 1 (1988) 52-55.
3. HAMAZINA, O. SH. Assessment of myeloperoxidase activity in patients with dermatophytoses against the background of immune therapy. Abstracts of 6th All-Russian Congress of Dermatologists and Venerologists M. (1989) 356-357.
4. MASLOV, A. K. A method of simulation of macrophage defect: Patent No 2105352. Discoveries Inventions 5 (1988).
5. MASLOV, A. K. and JUSCENKO, A. A. Assessment of functional activity of leprous macrophages. Archiv. Patologii. 11 (1988) 51-54.
6. MASLOV, A. K. and JUSCENKO, A. A. A method for prediction of leprosy relapse: Author's Certificate No 1636717 (USSR). Discoveries Inventions 11 (1991) 122.
7. MASLOV, A. K. and KAI.YANINA, O. V. A method of simulation of leprosy infection. Patent No. 102482. Discoveries Inventions 2 (1998).
8. MCKEEVER, P. E., WALSH, G. P., STORRS, E. E. and BALENTINE, J. D. Electron microscope of peroxidase and acid phosphatase in leprous and uninfected armadillo macrophages: a macrophage subpopulation contains peroxisomes and lacks bacilli. Am. J. Trop. Med. Hyg. 27 (1978) 1019-1929.
9. PIGAREVSKY, V. E. The new trend in the teaching on phagocytosis and nonspecific resistance. Archiv Patologii 2 (1977) 84-94.
10. UCHTTEL, I. YA. Macrophages in immunity. M. Medicine. 1978, 199 pp.