- Volume 68 , Number 1
- Page: 49–56
Human leukocyte antigens in forms of leprosy among japanese patients
ABSTRACT
Human leukocyte antigens (HLA) class II alleles were analyzed among Japanese leprosy patients to ascertain whether immunogenctic differences exist among the leprosy classification forms of Ridley and Jopling. Ninetγ-three unrelated Japanese leprosy patients (21 lepromatous, 24 borderline lepromatous, 17 mid-borderline, 26 borderline tuberculoid, 5 tuberculoid) and 114 healthy control subjects were investigated. The frequencies of HLA-DRB 1 * 1501, -DRB5*0101, -DQA1 *() 102 and DQB1 *0602 were significantly increased in all of the Japanese leprosy patients. The frequencies of HLA-DRB 1*0405, -DQA 1*03 and -DQB 1*0401 were significantly decreased in the Japanese patients after correction of the p value. Conversely, there were no significantly different distributions of the HLA-DRB 1, -DRB5, -DQA1, DQB1 alleles in the five subgroups of these patients. We conclude that HLA class II alleles were not associated with the form of leprosy. Other HLA, a non-HLA gene, and/or environmental factors may play a critical role in the different manifestations of leprosy.RÉSUMÉ
Les alleles des antigènes leukocytaires humains (HLA) de classe II de patients lépreux japonais furent analysés afin de vérifier si il n'existe pas de potentielles différences immuno-génétiques parmi les groupes définis selon la classification de Ridley et Jopling. Quatre vingt treize patients lépreux japonais sans liens familiaux (21 lépromateux, 24 lépromateux borderlines, 17 mid-borderlines, 26 tuberculoïdes borderlines et 5 tuberculoïdes) et 114 sujets contrôles en bonne santé furent examinés. Les fréquences des alleles HLA-DRB1*1501, -DRB5*0101, -DQA1*0102 et -DQB 1*0602 étaient toutes signilicativement augmentées chez tous les patients lépreux japonais. Les fréquences de HLA-DRB1 *0405, -DQA1*03 et -DQB 1*0401 étaient signilicativement diminuées chez, les patients japonais après correction de la valeur p. En revanche, les distributions des alleles de HLA-DRB 1, -DRB5, DQA1 et DQBI n'étaient pas significativement différentes lorsque les 5 sous-groupes de patients furent comparés. Nous concluons que les alleles des HLA de classe II ne sont pas associées avec les différentes formes de lèpre. D'autres HLA, des gènes autres que HLA et/ou des facteurs environmentaux devraient jouer un rôle critique dans les différentes manifestations de la lèpre.RESUMEN
Se estudiaron los alelos de los antígenos de histocompatibilidad humanos (HLA) en una problación de pacientes japoneses con lepra para saber si existen diferencias genéticas entre los diferentes grupos de la clasificación de Ridley y Jopling. Se incluyeron 93 pacientes con lepra (21 lepromatosos, 24 lepromatosos subpolares, 12 intermedios, 26 tuberculides subpolares, 5 tuberculoides) y 114 controles sanos. Mientras que las frecuencias de HLA-DRB 1 * 1501, -DRB5*0101, -DQA1*0102 γ-DQB1*0602 estuvieron significativamente elevadas en todos los pacientes con lepra, las frecuencias de los antígenos HLA-DRB 1*0405, -DQA 1*03 y DQB 1*0401 resultaron disminuidas después de corregir los valores de p. La distribución de los antígenos HLA-DRB1, -DRB5, -DQA1 y DQB1 fue similar en los pacientes de los cinco subgrupos. Concluimos que los alelos de los antígenos HLA clase II no están asociados con la forma clínica de la lepra. Tal vez otros antígenos HLA, antígenos no HLA o algunos factores ambientales, podrían jugar un papel crítico en las diferentes manifestaciones de la lepra.Leprosy is characterized by a spectrum of clinical manifestations, resulting from interactions between the host immune response and the invading Mycobacterium leprae (27). Although the immunobiology of leprosy has received much attention over the years, individual differences in resistance and response to the bacilli are still unknown. Furthermore, leprosy docs not occur in all patients who are infected with M. leprae, and contact with infectious cases does not always result in disease transmission. Twin, family and population studies have indicated that in humans host genetic factors are major determinants of susceptibility to leprosy (1,3-5,7,11-13, 17.23-26,29.30.32-36)
Previous studies, including ours, have demonstrated that leprosy is associated strongly with human leukocyte ANTIGENS (HLA)-DR2, especially the DRB 1*1501 and DRB5*0101 alleles of the DR2 group (12,25) yet few reports have been published on the relationship between HLA class II genotyping and the forms of leprosy, i.e., the classification of the disease ('7-25,31). To the best of the authors' knowledge, no reports have been published on the relationship between HLA class II genotypes and forms of leprosy in Japanese patients based on the classification of Ridley and Jopling 27). To ascertain whether immunogenctic differences exist among the forms of leprosy as defined by the Ridleγ-Jopling classification, we have analyzed HLA-DRB1, DRB5, DQA1 and DQB1 in Japanese patients with leprosy.
MATERIALS AND METHODS
Subjects. Ninetγ-three unrelated leprosy patients of Japanese ancestry were enrolled in our study and were partially identical with those patients previously reported on (l2). All patients had been hospitalized in the National Leprosarium Tama-Zensho-En in Tokyo, Japan. The diagnosis of leprosy was based on clinical manifestations, histological features and bacteriological examinations. According to the Ridleγ-Jopling criteria, the patients could be classified as 21 lcpromatous leprosy (LL), 24 borderline Icpromatous (BL), 17 mid-bordcrlinc (BB), 26 borderline tuberculoid (BT) and 5 tuberculoid leprosy (TT). We were unable to obtain samples from the TT patients because of the limited number of TT Japanese patients who met the Ridleγ-Jopling criteria. One-hundrcd-fourteen control subjects were also included in this study. The control subjects were healthy Japanese blood donors at the Saitama Medical Center and were all unrelated to each other.
All studies were approved by the Ethics Committee of the University of Tokyo. Informed consent was obtained from each subject before enrollment in the study, and the tenets of the Declaration of Helsinki were followed.
HLA serologic and genomic typing. A standard complement dependent microcytotoxicity test was used for typing the HLA-DR and HLA-DQ specificities (16). DNA analyses for the HLA-DRB1, DRB5, DQA1 and DQB1 alleles were performed using the polymerase chain reaction (PCR) single-strand conformation polymorphism (SSCP) method and the PCR-restriction fragment length polymorphism (RFLP) method (20,21)- DNA was extracted by using a standard phenol-chloroform extraction method for high molecular weight genomic DNA. After extraction, a PCR was conducted using group-specific primers of the second exon in accordance with methods presented in previous papers (2,10,14,19,22) . SSCP and RFLP analyses for HLA genotypings using PCR products were performed based on standard methods (20,21). Genomic DNA was extracted from peripheral blood leukocytes by phenol extraction. One u.iz of genomic DNA was amplified by PCR using DRB1, DRB5, DQA1 or DQB1 group-specific primers (2,10,14,19,22) .
Statistical analysis. A chi-squared analysis was conducted to determine the statistical significance of the differences between all patients and normal controls and among the five groups of patients; p values were corrected by multiplying the p value by the number of antigens tested, i.e., 20 or by 49 (the number of alleles investigated) for the comparison of patients with controls. The correction factor is 49 (number of alleles investigated) times 5 (number of clinical forms) = 245 for the comparison of each of the subgroups with the controls. The corrected p value was considered significant if less than 0.05. Odds ratios (OR) were estimated according to Woolf's formula, with Haldane's modification used when necessary.
RESULTS
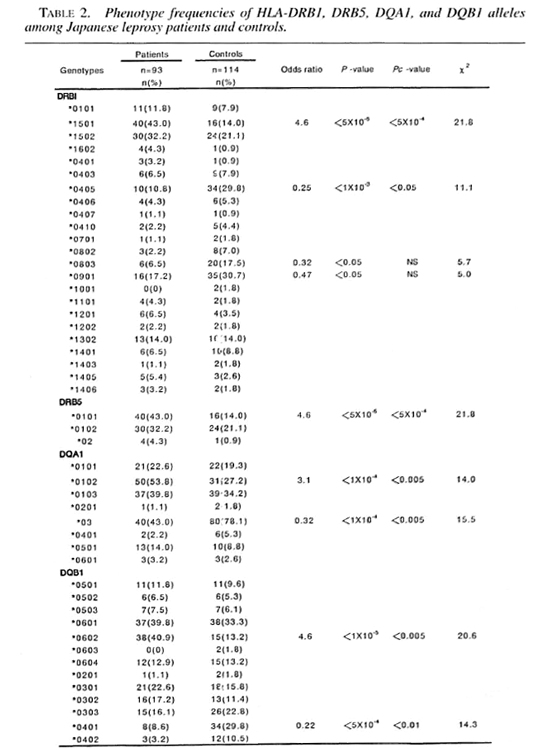
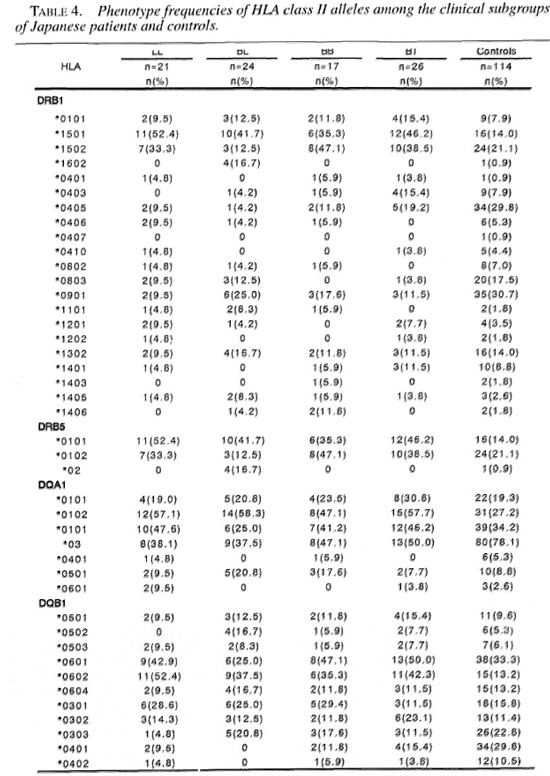
Tables 1 and 2 show the frequencies of HLA class II antigens and alleles in the Japanese leprosy patients and controls. The frequencies of HLA-DR2, DR4, DR53 and DQ4 were significant after the p value was corrected. The relative risks for HLA-DR2, DR4, DR53 and DQ4 were 4.2, 0.42, 0.31 and 0.21, respectively. The frequencies of HLA-DRB 1*1501, DRB1 *0405, DRB5*0101, DQA1*0102, DQA1*03, DQB 1*0602, DQB 1*0401 were significant after the p value was corrected. The relative risk for HLA-DRB 1 * 1501, DRB5*0101, DQA 1*0102 and DQB 1 *0602 were 4.6,4.6, 3.0 and 4.6, respectively. The frequencies of DQA 1 *03 and DQB 1*0401 were significantly lower in the leprosy patients. These alleles had relative risks of 0.32 and 0.22, respectively.
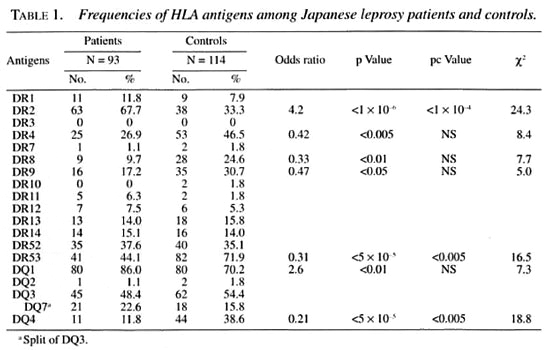
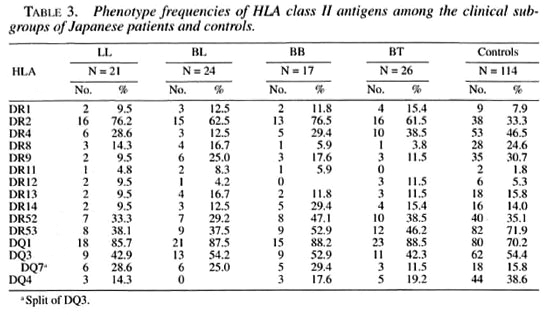
Tables 3 and 4 show the phenotype frequencies of HLA class II antigens and alleles in the Japanese leprosy patients classified according to Ridleγ-Jopling criteria. The distribution of HLA-class II antigens and HLA-DRB 1, DRB5, DQA1 and DQB1 alleles was not significantly different in any of the four groups of patients (TT data not shown).
DISCUSSION
Leprosy was reported to be positively associated with HLA-DR2 and DQI in several previous studies, including studies by the authors. At the genomic level, HLADRB1*1501, DRB5*0101, DQA1*0102 and DQB1 *0602 were positively associated with the leprosy patients in our present study. Rani, et al. (25) reported that the frequencies of HLA-DRB1 * 1501, DRB5*0101 and DQB 1*0601 were significantly increased in leprosy patients from North India as compared with normal controls after the p value was corrected. Our findings of a higher level in HLA-DRB 1*1501 and DRB5*0101 are consistent with Rani's data. However, Soebono and colleagues (31) reported no significant differences in the prevalence of 12 HLA-DRB 1 and 8 DQA1 alleles after p value correction between leprosy patients and control subjects from a Javanese population in Yogyakarta, Indonesia. These findings indicate that HLA-DRB 1*1501 and DRB5*0101 may play an important role in the pathogenesis of leprosy in some populations.
Several histological classifications for leprosy are available, such as the Ridleγ-Jopling criteria, WHO/MDT criteria and Japanese criteria (10,27,37,38). The question remains why, in spite of the same causative agent, do some people develop a more severe case of the disease than others. Many studies have indicated that HLA antigens are at least partially responsible for the form of leprosy a patient develops. Studies on families suggested an HLA-linked control of susceptibility to the form of leprosy ( 5,33,35) bud these studies had weak points. The family studies only looked for association with HLA scrotyping. HLA specificities, defined by serology, are now considered to include many genotypes at the DNA level. Those studies did not analyze the relationship between HLA class II genotypings and the five forms of leprosy (LL, BL, BB, BT and TT). Furthermore, leprosy has a long and asymptomatic incubation period before onset (15). Thus, the latency period is a very important consideration in families.
Only a few reports are available in the literature on the relationship between HLA genotyping and the form of leprosy. Our study is the first attempt to analyze the immunogenetic differences between the LL, BL, BB and BT types of leprosy in Japanese patients.
In this study, the frequencies of HLA class II antigens and alleles were not significantly different in the patient group. Some reports have been published on the relation ship of HLA and the form of leprosy based on Ridleγ-Jopling criteria. In 1982, van Eden, et al. (33) analyzed the relationship between HLA-A, -B, -C and DR antigens and the TT, BT, BL and LL types of leprosy in Surinamese patients. They reported that the frequency of HLA-DR3 in TT leprosy was higher than in other forms of leprosy. HLA-DR3, however, did not exhibit a significant correlation with the patient group. In 1987, Kim, et al. (13) reported HLA-A, -B, -C, -DR and DQ antigens in Korean patients with TT and LL leprosy, and no significant differences were found between TT and LL leprosy patients. In another report on TT and LL leprosy in Mexican patients, Gorodezky, et al. (7) did not find any significant differences in the presence of HLA-A, -B, -C and DR antigens. In 1992, Rani and colleagues studied the relationship between HLA-A, -B, -C, DR and DQ antigens in BB, BL and LL leprosy patients in North India (26). They did not find any significant differences in the presence of HLA-A, -B, -C, DR and DQ antigens after the p values were corrected.
No consistent pattern of association in any specific group was found in most of the previous population studies related to HLA class I and II antigens.
Few reports have been published on the relationship between HLA class II genotyping and the form of leprosy based on tbe Ridleγ-Jopling criteria. Rani, et al. (2S) reported that the frequencies of HLADRB1*1501 and DRB5*0101 were significantly increased and the frequency of HLA-DQB 1*0201 was significantly decreased in combined leprosy patients (LL + BL) as compared with TT leprosy patients from North India. Yet no differences in the distribution of HLA class II genotypings were observed between LL and BL patients. They did not, however, study HLA class II genotypings in BB and BT leprosy patients. Mchra, et al. reported that the frequencies of HLA-DRB 1*1501 and DRB 1*1502 were increased in Indian patients with TT leprosy compared to normal controls (17), but they did not study HLA class II genotypings in LL, BL, BB and BT leprosy patients. Soebono, et al. reported no significant differences in the prevalence of HLA-DRB 1 and DQA1 alleles between LL/BL and BT/TT leprosy patients from a Javanese population in Yogyakarta, Indonesia (31). None of these three studies examined the relationship between HLA class II genotyping and the five forms of leprosy (LL, BL, BB, BT and TT). Thus, further detailed studies must be conducted on a large number of patients in different populations.
We conclude in this study that HLA class II alleles arc not associated with the form of leprosy. Therefore, other HLA, a non-HLA gene, and/or environmental factors may play a critical role in the differing manifestations of leprosy (6.9.28).
REFERENCES
1. ABEL, L., SANCHETS, O. F, OBERTI, J., THUC, V. N., HOA, L. V., LAP, V. D., SKAMENE, E., LAGRANGE, R H. and SCHURR, E. Susceptibility to leprosy is linked to the human NRAMP1 gene. J. Infect. Dis. 177(1998) 133-145.
2. AKIVAMA, K., YOSHII, T. and ISHIYAMA, I. [Investigation of the medium composition for PCR-SSCP analysis of the HLA-DQA gene region.] Jpn. J. Electroph. 37 (1993) 41-44.
3. BALE:, U. M., MEHTA, M. M., CONTRACTOR, N. M., BHATIA, H. M. and KOTICHA, K. K. HLA antigens in leprosy patients. Tissue Antigens 20 (1982) 141-143.
4. CHAKRAVARRTI, M. R. and VOGEL, F. A twin study on leprosy. In: Topics in Human Genetics, Vol. I. Becker, P. E., et al., eds. Stuttgart: Thieme Verlag, 1973, pp. 1-23.
5. DESSOUKEY, M. W., EL-SHIEMY, S. and SALI.AM, T. HLA and leprosy segregation and linkage study. Int. J. Dermatol. 35 ( 1996) 257-264.
6. DORMAN, S. E. and HOLLAND, S. M. Mutation in the signal-transducing chain of the interferon gamma receptor and susceptibility to mycobacterial infection. J. Clin. Invest. 101 (1998) 2364-2369.
7. GORODEZKY, C, FLORES, J., AREVALO, N., CASTRO, L., SII.VA, A. and RODRIGUEZ, O. Tuberculoid leprosy in Mexicans is associated with HLA-DR3. Lepr. Rev. 58 (1997) 401-406.
8. GOTO, M., SUZUK, M., KITAJIMA, S. and IMAIZUMI, M. [Changes and present status of a Japanese National Leprosarium-analysis of smear positive rate and relapse in Hosizuka-Keiaien between 1972-1991.] Jpn. J. Lepr. 62 (1993) 1-12.
9. HACKAM, D. J., ROTSEIN, O. D., ZHANG, W., GKUENHEID, S., GROS, P. and GRINSTEIN, S. Host resistance to intracellular infection: mutation of natural resistance-associated macrophage protein 1 (Nrampl) impairs phagosomal acidification. J. Exp. Med. 188 (1998) 351-364.
10. ISLAM, S. M. M., NUMAGA, J., FUJINO, Y., HIRATA, R., MATUKI, K., MAEDA, H. and MASUDA, K. HLA class II genes in Vogt-Koyanagi-Harada disease. Invest. Ophthalmol. Vis. Sci. 35 (1994) 3890-3896.
11. IZUMI, S., SUGIYAMA, K., MATSUMOTO, Y. and OHKAWA, S. Analysis of the immunogenetic background of Japanese leprosy patients by the HLA system. Vox Sang. 42 (1982) 243-247.
12. JOKO, S., NUMAGA, J., FUJINO, Y, MASUDA, K., HIRATA, R. and MAEDA, H. [HLA-DR2 alleles and uveitis in leprosy.] Jpn. J. Lepr. 64 (1995) 112-118.
13. KIM, S. J., CHOI, I. H., DAHLBERG, S., NISPEROS, B., KIM, J. D. and HANSEN, J. A. HLA and leprosy in Koreans. Tissue Antigens 29 (1987) 146-153.
14. KIMLURA, A. and SASAZUKI, T. Eleventh International Histocompatibility Workshop reference protocol for the HLA DNA-typing technique. In: HLA 1991. Tsuji, K., Aizawa, M. and Sasazuki, T, eds. Oxford: Oxford University Press, 1992, pp. 397-419.
15. LEVIS, W. R., SCHUMAN, J. S., FRIEDMAN, S. M. and NEWEIEI.D, S. A. An epidemiologic evaluation of leprosy in New York City. JAMA 247 (1982) 3221-3226.
16. MAEDA, H. and JUJI, T. A new B-cell alloantigen, TB21, coded for in the HLA-D/DR region. Tissue Antigens 20 (1982) 327-334.
17. MEHRA, N. K., RAJAI.INGAM, R., MITRA, D. K., TANEJA, V. and GlPHART, M. J. Variants of HLA-DR2/DR51 group haplotypes and susceptibility to tuberculoid leprosy and pulmonary tuberculosis in Asian Indians. Int. J. Lepr. 63 (1995) 241-248.
18. MIYANAGA, K., JUJI, T, MAEDA, IL, NAKAJIMA, S. and KOUAYASHI, S. Tuberculoid leprosy and HLA in Japanese. Tissue Antigens 18 (1981) 331-334.
19. MOI.KENTIN, J., GROSKI, J. and BAXTER-LOWE, L. A. Detection of 14 HLA-DQB1 alleles by oligotyping. Human Immunol. 31 (1991) 114-122.
20. NOMURA, N., OTA, M., TSUJI, K. and INOKO, H. HLA-DQB1 genotyping by modified PCR-RFLP method combined with alleles-specilic primers. Tissue Antigens 38 (1991) 53-59.
21. ORITA, M., IWAHANA, H., KANAZAWA, H., HAYASIII, K. and SEKIYA, T. Detection of polymorphisms of human DNA by gel electrophoresis as singlestrand conformation polymorphisms. Proc. Natl. Acad. Sci. U.S.A. 86 (1989) 2766-2770.
22. OTA, M., SEKI, T., FUKOSHTMA, II., TSUJI, K. and INOKO, H. HLA-DRB1 genotyping by modified PCR-RFLP method combined with group-Specific 31.primers. Tissue Antigens 39 (1992) 187-202.
23. OTTENHOFF, T. H. M., GONZALEZ, N. M, DE: VRIES, R. R. P., CONVII, J. and VAN ROOD, J. J. Association of HLA specificity LB-EI 2(MB 1 ,DC 1 ,MTI) with lepromatous leprosy in a Venezuelan popula32.tion. Tissue Antigens 24 (1984) 25-29.
24. POLLACK, M S., OHM; , C, PANDEY, J. and REICHERT, E. HLA antigen frequencies and HLA and Gm haplotype segregation in Filipino leprosy patients in Hawaii. Dis. Markers 3 (1985) 119-129.
25. RANI, R., FERNANDEZ-VINA, M. A., ZAHEER, S. A., BEENA, K. R. and STASTNY, P. Study of HLA class II alleles by PCR oligotyping in leprosy patients from North India. Tissue Antigens 42 (1993) 34.133-137.
26. RANI, R., ZAHEER, S. A. and MUKIIERJEE, R. DO human leukocyte antigens have a role to play in differential manifestations of multibacillary leprosy: a study on multibacillary leprosy patients .from North India. Tissue Antigens 40 (1992) 124-127.
27. RIDLEY, D. S. and JOPUNG, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34 (1966) 255-273.
28. ROY, S., McGUIRE, W., MASCIE; TAYLOR, C. G., SAHA, B., HAZRA, S. K., HILL, A. V. and KWIATKOWSKI, D. Tumor necrosis factor promoter polymorphism and susceptibility to lepromatous .leprosy. J. Infect. Dis. 176 (1997) 530-532.
29. SCHAUE, V, RYAN, S., SCOI.I.ARD, D., JONASSON, O., BROWN, A., NELSON, K., SMITH, T. and VITHAYASAI, V. Leprosy associated with HLA-DR2 and DQwl in the population of northern Thailand. Tissue Antigens 26 (1985) 243-247.
30. SERJEANSTON, S. W., VAIDYA, M. C, CHAN, S. H., JUJI, G. T, MEHRA, N. K., NAIK, S. and SASAZUKI, T. Leprosy. In: Proceedings of the Second Asia and Oceania Histocompatibility Workshop Conference. Simons, M. J., and Tait, B. D., eds. Toorank, Victoria, Australia: Immunopublishing, 1983, pp. 379-401.
31. SOEHONO, H., GIPHART, M. J., SCHREUDER, G. M. T, KI.ATSER, P. R. and DE VRIES, R. R. P. Associations between HLA-DRB1 alleles and leprosy in an Indonesian Population. Int. J. Lepr. 65 (1997) 190-196.
32. TODD, J. R., WEST, B. C. and McDONALD, J. C. Human leukocyte antigen and leprosy: study in northern Louisiana and review. Rev. Infect. Dis. 12(1990) 63-74.
33. VAN EDEN, W., DE VRIES, R. R. P., D'AMARO, J., SCHREUDER, I., LEIKER, D. L. and VAN ROOD, J. J. HLA-DR-associated genetic control of the type of leprosy in a population from Surinam. Hum. Immunol. 4 (1982) 343-350.
34. VAN EDEN, W., DE VRIES, R. R. P., MEHRA, N. K., VAIDYA, M. C, D'AMARO, J. and VAN ROOD, J. J. HLA segregation of tuberculoid leprosy: confirmation of the DR2 marker. J. Infect. Dis. 141 (1980)693-701.
35. VAN EDEN, W, GONZALEZ, N. M., DE VRIES, R. R. P., CONVIT, J. and VAN ROOD, J. J. HLA-linked control of predisposition to lepromatous leprosy. J. Infect. Dis. 151 (1985)9-14.
36. VAN EDEN, W., MEHKA, N. K., VAIDYA, M. C, D'AMARO, J., SCHREUDER, G. M. T. H. and VAN ROOD, J. J. HLA and sporadic tuberculoid leprosy: a population study in Maharashtra, India. Tissue Antigens 18 (1981) 189-194.
37. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1998. Tech. Rep. Ser. 768.
38. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.D.; Department of Ophthalmology, Faculty of Medicine, University of Tokyo, Japan.
2. M.D.; Department of Ophthalmology, Faculty of Medicine, University of Tokyo, Japan.
3. M.D., Department of Ophthalmology, Faculty of Medicine, University of Tokyo, Japan.
4. M.D., National Leprosarium Tama-Zensho-En, Tokyo, Japan.
5. M.D., Blood Transfusion Service, Saitama Medical Center, Saitama Medical School, Japan.
Reprint requests to: Jiro Numaga, M.D., 3-2-1-214 Nishigahara, Kita-ku, Tokyo 114-0024, Japan. Fax: 81-3-3915-9044; e-mail: jnumaga-@mub.biglobe.ne.jp
Received for publication on 14 September 1999.
Accepted for publication in revised form on 19 January 2000.