- Volume 68 , Number 1
- Page: 57–62
Epidemiology of leprosy in Taiwan; its pattern in children
ABSTRACT
OBJECTIVE: To study the epidemiology of leprosy in children in Taiwan.SETTING: Taiwan, with a population increase f rom 3.3 to 21.7 million, several tides of immigration and national leprosy control programs, f rom 1910 to 1997.
DESIGN: To collect and analyze the documents of Taiwan leprosy surveys and charts of the National Leprosy Control Center.
PATIENTS AND MEASUREMENTS: Cumulative and new number of all-age and pediatricage patients, prevalence rates, new case detection rates, and results of skin bacterial smears.
RESULTS AND CONCLUSIONS: The prevalence rates of all-age leprosy ranged between 1.54 and 3.22 per 10,000 population. The proportion of children among all-age patients reached the highest of 4.93% in 1966, dropping to 0% in 1984 and thereafter, until 1988 and 1991 when two and one pediatricagc patients appeared, respectively, following the influx of immigrants f rom leprosyendemic countries. The rise and fall of new patients younger than 15 years and 15 years or older were slightly correlated (r = 0.935, p <0.001). Detection and confirmation of leprosy in children are usually belated. Physicians should still be acquainted with the clinical diagnosis of leprosy since sporadic cases of leprosy can reappear, particularly among children coming f rom endemic countries.
RÉSUMÉ
OBJECTIF: Etudier l'épidémiologie de la lèpre che/, les enfants de Taïwan.CADRE DE L'ETUDE: Taïwan, qui a connu une augmentation de population de 3,3 à 21,7 millions, avec plusiers vagues d'immigration et des programmes nationaux de contrôle de la lèpre, de 1910 à 1997.
ELABORATION DE L'ETUDE: Collecter et analyser les documents issus des enquêtes epidémiologiques taïwanaises concernant la lèpre et les tableaux du Centre National du Contrôle de la Lèpre.
PATIENTS ET VARIABLES MESURÉES: Nombre cumulé et nombre incident de patients pédiatriques et de tous âges, les taux de prévalence, les taux de détection des nouveaux cas et les résultats d'examen de suc dermique.
RESULTATS ET CONCLUSIONS: Les taux de prévalence pour la lèpre, quelqu'en soit l'âge, se situaient entre 1.54 et 3.22 pour 10,000 personnes de cette population. La proportion des enfants parmi les patients de tous âges atteignit un maximum de 4.93% en 1966, baissant drastiquement à 0% en 1984 et ensuite, jusqu'en 1988 et en 1991, lorsque deux patients pédiatriques et un patient pédiatrique apparurent, respectivement, probablement à la suite de vagues d'immigrants provenant de pays où la lèpre est restée endémique. La réémergeance puis la diminution de nouveaux patients de moins de 15 ans et de 15 ans et plus était faiblement corrélée (r = 0.935, p <0.00I ). La détection et la confirmation de la lèpre chez les enfants sont le plus souvent tardives. Les médecins devraient se maintenir au courant du diagnostic clinique de la lèpre du fait de la ré-apparition toujours possible de cas sporadiques de lèpre, en particulier chez les enfants venant de pays endémiques.
RESUMEN
OBJETIVO: El estudio de la epidemiología de la lepra en niños en Taiwan.ESCENARIO: Taiwan de 1910 a 1997, con un aumento en la población de 3.3 a 21.7 millones, varias oleadas de inmigración y programas de control de la lepra.
DISENO: Colección y análisis de los documentos de los programas de control y vigilancia de la lepra del Centro Nacional para el Control de la Lepra.
PACIENTES Y MEDICIONES: Número acumulado y casos nuevos de pacientes con lepra, incluyendo los pediátricos, tasas de prevalência, tasas de detección de nuevos casos, resultados bacteriológicos en linfa cutánea.
RESULTADOS Y CONCLUSIONES: Las tasas de prevalência de la lepra, incluyendo a los pacientes de todas las edades, oscilaron entre 1.54 y 3.22 por 10,000 habitantes. La proporción de niños entre todos los pacientes fue de 4.937o en 1966 y de 0% en 1984 y posteriormente, hasta 1988 y 1991 cuando, siguiendo al INFLUJO de inmigrantes de países endémicos, aparecieron dos casos pediátricos. El aumento y disminución de los nuevos pacientes menores de 15 años y de los pacientes de 15 años o mayores estuvieron ligeramente correlacionados (r = 0.035, p <0.001). La detección y confirmación de la lepra en niños generalmente correlacionan bien. Los médicos deben seguir preparados para reconocer y diagnosticar la enfermedad ya QUE pueden reaparecer casos esporádicos de lepra, sobre todo en niños que vienen de países con la enfermedad endémica.
Leprosy could have been transmitted to Taiwan during the 16th and 17th centuries when Chinese, Portuguese, Dutch and Spanish landed on the island (1,2). The first leprosy asylum in Taiwan was built in 1736 to accommodate homeless patients with leprosy (2). From 1895 to 1945, two leprosaria were established and a compulsory isolation policy was implemented (2 and Chao, Y. F, Lai H. H., Su H. Y.: Past and present status of leprosy (Hansen's disease) on Taiwan; a successful leprosy control on Taiwan. Report to ILEP Meeting, Liege, Belgium, Dec. 9-12, 1998). From 1945 to 1949, after World War II multitudes of soldiers and civilians with leprosy moved from mainland China to Taiwan. In the late 1950s, Dapsone treatment and BCG vaccination programs were launched. An official nationwide leprosy control program was implemented in 1962, and has continued until the present, enforcing free-of-charge case detection, treatment, surveillance, care and education. Commencing in 1982, the World Health Organization multidrug therapy (WHO/MDT) program was introduced. From the late 1980s, more than 200,000 laborers from South-East Asian countries immigrated to Taiwan, bringing in a considerable number of people with leprosy.
To study the epidemiology of leprosy in the children of Taiwan, we collected and analyzed the documents of the Taiwan leprosy surveys carried out from 1910 to 1997, and the charts of the National Leprosy Control Center. The results of our analyses are reported here.
MATERIALS AND METHODS
The documents of the serial Taiwan leprosy surveys carried out during the past 87 years (1910-1997) were collected and analyzed. The reports and charts of the National Leprosy Control Center (Lo-Sheng Leprosarium) were reviewed and analyzed. All of the patients were registered and followed until 1978 and thereafter, when some of those designated as cured were released from the registry.
The yearly numbers of new all-age and pediatric-age (under 15 years) patients, the cumulative numbers of all-age and pediatric-age patients, and the results of bacterial examination of biopsies and/or skin smears were analyzed. The bacterial study was judged as positive if in 100 oil-immersion fields there was one or more Mycobacterium leprae bacilli and the bacterial index (BI) was 1+ or greater on the Ridley scale (9). All patients with a positive skin smear were termed multibacillary (MB), including mainly mid-borderline (BB), borderline lepromatous (BL), and lepromatous (LL) cases with the Ridlcγ-Jopling (R-J) classification or borderline (B) and lepromatous (L) with the Madrid classification (6,14) . Those with negative skin smears were termed as paucibacillary (PB), including indeterminate (I), tuberculoid (TT) and borderline tuberculoid (BT) (R-J) or indeterminate (I) and tuberculoid (T) (Madrid) cases (6,10,14) . Any case belonging to these types but having a positive skin smear was classified as MB (10,11) The prevalence rate was expressed as the number of patients per 10,000 population. New case detection ratewas expressed as the number of new patientsdetected each year per 100,000 population. The population in Taiwan listed each yearwas the numbcr of persons at the end of year.A report on a cohort of 157 ali-age patientsand their families was also reviewed (3).
Statistical analysis. The yearly number of new patients younger than 15 years of age and of 15 years or older was assessed using Spearman's correlation. A p value of <0.05 is considered to be statistically significant.
RESULTS
All-age patients. The leprosy surveys revealed 811 patients in 1910. The cumulative number of patients was 641 in 1918, 756 in 1926, and 1084 in 1930, with prevalence rates of 2.46, 1.75, 1.78, and 2.32 per 10,000 population for 1910-1930, respectively. The cumulative number decreased to 850 in 1935, but increased again in the late 1940s after World War II. It rapidly increased to 1993 in 1959 and to 4942 in 1977. Starting in 1978, those patients designated as cured were released from the registry (Table 1). The number of new all-age cases in 1930 was 30, with a new case detection rate of 0.64 per 100,000 population (Table 1).
The number of new cases increased to the highest of 379 with a new case detection rate of 3.33 per 100,000 population in 1962 (Fig. 1). Following the implementation of the National Leprosy Control Program in 1962, the number of new leprosy cases started to drop, especially after the introduction of the WHO/MDT program in 1982. Commencing in 1988, more new leprosy cases appeared and the number of cases fluctuated. The percentage of MB patients among the new all-age patients in 1986 to 1997 range d from 40% to 86.7% (Table 2). The male-tofemale patient ratio in Taiwan was the highest, 3.96, in 1961 and then decreased to 2.61 in 1997.
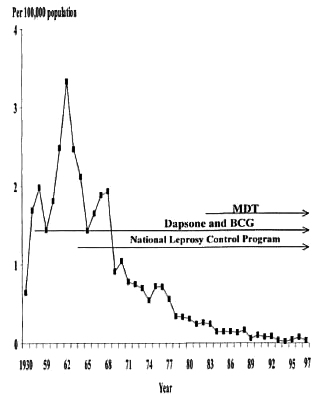
Fig. 1. New all-age leprosy case detection rates in Taiwan from 1930 to 1997, and implementation of dapsone treatment and BCG vaccination, National Leprosy Control Program and multidrug therapy (WHO/MDT).
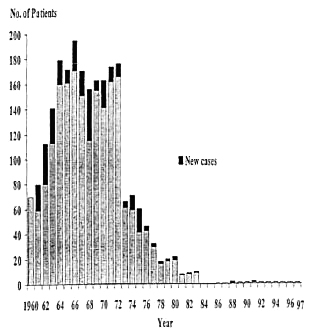
Fig. 2. Cumulative number of pediatric-age leprosy patients in Taiwan, showing a dramatic decrease in 1973 and 0% during 1984-1987. Two new cases and one new case appeared in 1988 and 1991, respectively.
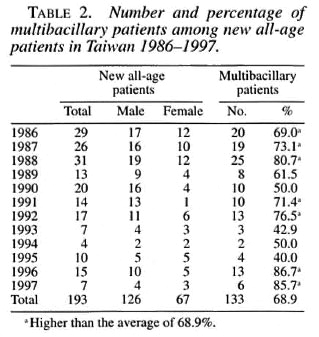
Pediatric-age patients. The number of new patients under 15 years of age was 21 in 1961, reaching the highest of 41 in 1968. It then decreased but fluctuated. The highest cumulative number of pediatric-age patients was 195 in 1966. Beginning in 1976, the decrease was dramatic, reaching to 0 for 4 years (1984 to 1987) (Table 1). Two new pediatric cases appeared in 1988, then another case in 1991 (Table 1). Of these three cases, one was a 14-year-old girl who had immigrated from Indonesia together with her family (Fig. 3A). Another was an 8-year-old boy who had immigrated from Myanmar to Taiwan with his family in 1987 (Fig. 3B). The percentage of children among all new patients reached its highest of 16.4% in 1975 (Table 1). The yearly number of new leprosy patients under age 15 years and 15 years or older correlated very well (r = 0.935, p <0.001) (Fig. 4).
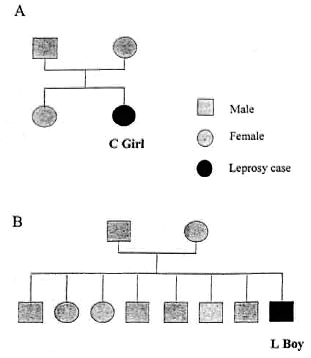
Fig. 3. A = C girl, born in Indonesia, 1974. Immigrated to Taiwan with her family in 1985. Diagnosed as BB leprosy in 1988. B = L Boy, born in Myanmar in 1983. Immigrated to Taiwan with his family of 10 in 1987. Diagnosed as BB leprosy in 1991.

Fig. 4. The number of new patients <15 years old and >15 years old in Taiwan, 1960-1997.
DISCUSSION
A leprosy survey on a total population of 4,679,066 carried out in 1930 in Taiwan revealed that the prevalence rate among aborigines was very low, 0.58/10,000 population, compared with 2.73/10,000 among Taiwanese, 3.83/10,000 among mainland Chinese, and 1.69/10,000 among Japanese, indicating that leprosy could have been introduced to Taiwan from abroad (: and Chao, Y. F, Lai H. H., Su H. Y: Past and present status of leprosy (Hansen's disease) on Taiwan; a successful leprosy control on Taiwan. Report to ILEP Meeting, Liege, Belgium, Dec. 9-12, 1998). Leprosy in Taiwan, with a peak prevalence of 3.22/10,000 population, was not a serious problem compared with that in Myanmar, where the prevalence was 8.62/10,000 population in 1973-1977 and 26.82/10,000 in 1988-1992 (10.14)
In the Taiwan leprosy registry, quite a number of cured patients have been included in the cumulative number of patients. This explains why the prevalence rate still remains high. Following the Second World War, two million soldiers and civilians moved from mainland China to Taiwan, bringing in a large number of" male leprosy patients. This special circumstance explains why the male-to-female ratio of leprosy patients in Taiwan was high, up to 3.96 in 1961. Of the 157 all-age inhabitant patients in southern Taiwan studied in 1987, the male-to-fcmale ratio was 1.9, attesting to the above explanation (3). During 1986 to 1997, we found that 40% to 87% of the new leprosy patients in Taiwan were MB cases, while only 12.5% of new patients were MB cases in leprosγ-endemic countries (10,14) The high proportion of MB leprosy with a low proportion of children among new cases in Taiwan suggests that leprosy has been declining, a phenomenon observed by Ramasoota and Intaratitaya (8).
Identification of points of onset of leprosy exposure and infection among children has been always difficult. The two new leprosy children who immigrated to Taiwan from Indonesia and Myanmar could have been infected before coming to Taiwan. It is important to note that for each of them there was only one leprosy case among their family members. Selvasekar, et al. (13) inferred that younger children are at the greatest risk for M. leprae infection. A cohort study of 157 all-age leprosy patients in southern Taiwan in 1987 revealed that 21 (13.4%) had onset before 14 years of age, 6 (3.8%) at age 5-9 years, and 15 (9.6%) at age 10-14 years. Two patients (1.3%) had their diagnoses confirmed at age 5-9 years, and 9 (5.7%) at age 10-14 years. The time lag before confirmation of diagnosis was 1 to 16 years, with an average of 3.2 years (3).
The Taiwan experience documented that elimination of leprosy is feasible and possible. M. leprae infections occur in children and the detection and confirmation are usually belated. Measures aimed at early detection and confirmation of leprosy need to be developed. Children living in or coming from high-risk regions should be quarantined and given special attention (4,5). Most children had only a single lesion (13). Children with a hypopigmented macule should arouse suspicion of leprosy, either of indeterminate, tuberculoid, borderline tuberculoid, or the borderline type (12). Active case finding and self-reporting programs of leprosy can be integrated into the health services and screening of immigrants from endemic countries.
Acknowledgment. The authors are grateful to all the staff of the Lo-Sheng Leprosarium and the Executive Yuan Department of 1 lealth who contributed to the data collection. We are also grateful to Dr. W. Y Shaw for his invaluable advice on statistical analysis.
REFERENCES
1. BROWNE, S. G. The history of leprosy. In: Leprosy. 2nd edn. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1994, pp. 1-14.
2. CHAO, Y. F. The leprosy problems in Taiwan. Lepr. Rev. 39(1968) 107-109.
3. CHOU, P., TSAI, P. F. and SOONG, L. N. Analysis of factors related to treatment and prognosis of leprosy patients in southern Taiwan. Chin. Med. J. (Taipei) 52 (1993) 1-8.
4. DAVEY, T. F. and REES, R. J. W. The nasal discharge in leprosy; clinical and bacteriological aspects. Lepr. Rev. 45(1974) 121-134.
5. FINE, P. E. M. Leprosy; the epidemiology of a slow bacterium. Epidemiol. Rev. 4(1982) 161-188.
6. INTERNATIONAL CONGRESS OI LEPROSY, MADRID 1953. Report of the Committee on Classification. Int. J. Lepr. 21 (1953)504-516.
7. MYINT, T. and HTOON, M. T. Leprosy in Myanmar; epidemiological and operations changes, 1958-92. Lepr. Rev. 67 ( 1996) 18-27.
8. RAMASOOTA, P. and INTARATITAYA, T. Progress and impact of multidrug therapy (MDT) implementation to leprosy control in Thailand. Jpn. J. Lepr. 64 (1995)214-219.
9. RIDLEY, D. S. and HII.SON, G. R. F. A logarithmic index of bacilli in biopsies. I. Method. Int. J. Lepr. 35(1967)184-186.
10. RLDLEY, D. S. and JOPLING, W. H. A classification of leprosy for research purposes. Int. J. Lepr. 33 (1962)119-128.
11. RRDLEY, D. S. and JOPLING, W. II. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34 (1955) 255-273.
12. SEHGAL, V. N. and CHAUDHKY, A. K. Leprosy in children; a prospective study. Int. J. Dermatol. 32 (1993)194-197.
13. SELVASEKAR, A., GEETHA, J., NISHA, K., MANT-MOZHI, N., JE SUDASAN, K. and RAO, R. P. S. Childhood leprosy in an endemic area. Lepr. Rev. 70(1999)21-27.
14. WHO EXPERT COMMUTÉE ON LEPROSY. Seventh report. Geneva: World Health Organization, 1997.
1. M.D., Ph.D., Department of Pediatrics, National Taiwan University College of Medicine, Taipei, Taiwan.
2. M.D., Taiwan Provincial Lo-Sheng Leprosarium, Taipei, Taiwan.
3. M.D.; Taiwan Leprosy Relief Association, Taipei, Taiwan.
4. Taiwan Leprosy Relief Association, Taipei, Taiwan.
5. Ph.D., National Yang-Ming University Institute of Public Health, Taipei, Taiwan.
6. M.D., Executive Director, Yuan Department of Health, Taipei, Taiwan.
Reprint requests to Dr. Lue at the above address or FAX 886-2-341-2601; email hclue@ha.mc.ntu.edu.tw
Received for publication on 10 August 1999.
Accepted for publication in revised form on 11 January 2000.