- Volume 68 , Number 2
- Page: 136–42
Induction of lepromin positivity and immunoprophylaxis in household contacts of multibacillary leprosy patients: a pilot study with a candidate vaccine, Mycobacterium w
ABSTRACT
We screened 487 household contacts of multibacillary (MB) patients for evidence of disease and their lepromin status. From the 444 results available, 302 (68.02%) were lepromin positive and 142 (31.98%) were lepromin negative on initial testing. The initial lepromin status as assessed in the group of 54 contacts having disease at the outset showed 24 out of 46 (52.2%) to be lepromin positive and 22 of 46 (47.8%) to be lepromin negative. In the same group, among 24 lepromin positives, 22 (91.7%) had paucibacillary (PB) and 2 (8.3%) had multibacillary (MB) disease; among the lepromin negatives, 12 (54.5%) had PB and 10 (45.5%) had MB disease. Out of 72 initially lepromin-negative contacts administered Mycobacterium w vaccine and followed up. the cumulative percentages show that 53 (73.6%) converted lo positivity after a single dose, 10 (87.5%) after a second dose and 67 (93.1%) after the third dose. The incidence of new cases with leprosy was 8 out of 231 (3.46%) among Iepromin- positive contacts and 5 out of 93 (5.38%) among lepromin-negative contacts administered Mycobacterium w vaccine. Among 231 lepromin-positive contacts, the new cases occurred in those with a 1+ and 2+ lepromin response only, and no case occurred among 51 contacts with a 3+ lepromin response. The incidence among lepromin-positive contacts in this study (3.46%) was similar to the observations in two other studies: 3.2% by Dharmendra, et al. and 6.9% by Chaudhary, et at. However, the incidence among lepromin-negative contacts administered Mycobacterium w vaccine was significantly lower than that observed among lepromin-negative contacts not administered any vaccination in the other two studies (14.1 % by Dharmendra, et al. and 29.0% by Chaudhary, et al.). To conclude, although a study of small sample size, the preliminary evaluation indicates that administration of Mycobacterium w vaccine seems to have the potential lo reduce the incidence of leprosy among household contacts of leprosy patients. More explicit results about the vaccine will be available f rom the ongoing field trials in Kanpur Dehat in the near future.RÉSUMÉ
Nous avons étudie 487 personnes habitant sous le même toil que tics patients multibacillaires (MB) pour la présence de signes tic la maladie et le status tie ces patients via-à-vis tie la lépromine. A partir tic 444 données disponibles. 302 (68.02%) étaient positifs a la lépromine, et 142 (31.98% ) étaient négatives lors du premier test. Le statut vis à vis de la lépromine. évalué chez, le groupe de 54 personnes contactes montrant des signes de lèpre au début de l'étude, était de 24/46 (52.2%) positifs et 22/46 (47.8%) négatif à la lépromine. Chez ce même groupe, parmi les 24 cas positifs à la lépromine, 22 (91.7%) souffraient de lèpre pau- cibacillaire (PB) et 2 (8.3%) souffraient de lèpre multi- baeillaire (MB); entre les négatives à la lépromine, 12 (54.5%) souffraient de lèpre paueibacillaire (PB) et 10 (45.5%) souffraient de lèpre MB. Parmi les 72 personnes contactes initialement négatives à la lépromine, chez lesquelles un vaccin utilisant Mybaclerium vf fut administré, les pourcentages cumulés pendant le suivi ont montré que 53 (73,6%) devenaient positifs au test à la lépromine après la première injection, 63 (87,5%) après la deuxième imjection et 67 (93,1%) après l'injection de la troisième dose. L'incidence de nouveau cas de lèpre était de 8 sur 231 (3,46%) parmi les contacts positifs a la lépromine et 5/93 (5,38%) chez les contacts négatifs à la lépromine chez lesquels un vaccin à My- cobacterium w a été administré. Parmi les 231 contacts positifs à le lépromine, les nouveau cas ont été observés chez ceux montrant une réponse faible ( 1+) à modérée (2+). Aucun nouveau cas ne fut enregistré parmi les contacts montrant une fort réponse (3+). Dans cette étude, l'incidence de lèpre (3.46%) parmi les contacts positifs à la lépromine est comparable à celles observées dans deux autres études: 3.2% pour celle de Dharmen- dra et coll. et 6.9% pour celle de Chaudhary et coll. Cependant, l'incidence parmi les contacts négatifs à la lépromine chez qui un vaccin à Mycobacterium w fut administré était significativement plus basse que celles observées parmi les contacts négatifis à la lépromine n'ayant pas reçu de vaccination (14.1% pour celle de Dharmendra et coll. et 29.0% pour celle de Chaudhary et coll.). Pour conclure, cette étude préliminaire, quoique de faible échantillonnage, indique que l'administration du vaccin basé sur Mycobacterium w semble présenter le potentiel de rédnire l'incidence de la lèpre parmi les personnes vivant sous le même toit que des patients lépreux. Des résultats plus définitifs sur l'impact de ce vaccin seront publiés prochainement à partir d'essais vaccinaux sur le terrain, en cours dans la province de Dehat Kanpur.RESUMEN
Se investigo la presencia de enfermedad y la reactividad a la lepromina en 487 contactos familiares de pacientes con lepra multibacilar (MB). I)e los 444 resultados con los que se puilo contar, 302 (68.02% ) lucrou lepromino-posilivos y 142 (31.98% ) lepromino- negativos en la prueba inicial. Entre los contactos coil evidencias de enfermedad, 24 de 46 (52.2% ) fueron lepromino-posilivos y 22 de 46 (47.8%) lueron lepromino-negativos. En el mismo grupo. 22 de 24 (91.7%) fueron paucibacilers (PB) y 2 (8.3%) lueron multibacilers (MB): entre los lepromino-posilivos. 12 de 24 (54.5%) pacientes lepromino-posilivos lueron paucibacilers (PB) y 10 (45.5%) fueron multibacilares (MB). De 72 contados inicialmente lepromino-negativos. a quienes se vacunó con Mycobacterium w. 53 se tornaron lepromino-posilivos después de una dosis de la vacuna (73.6% ). 10 después de la segunda dosis (87.5% acumulativo), y 4 después de la torcera dosis (93.1 % acumulativo), l.a incidência de nuevos casos do lapra lue del 3.46% (8 do 231 ) entre los contactos lepromino-posilivos y del 5.38% (5 de 93) entre los contactos lepromino-negativos vacunados con Mycobacterium w. Entre los 231 contactos lepromino-posilivos. los casos nuevos ocurrieron solo en aquellos contactos con una respuesta débil a la lepromin (1+ y 2+). Ningún caso nuevo ocurrió entro los 51 contactos con respuostas fuertos a la lepromina (3+). l.a incidência de la enfermedad entre los contactos lepromino-posilivos en este estúdio (3.46%) lue similar a la observada en oiros tios estúdios: 3.2Vi por Dharmendra. et al. y 6.9% por Chaudhary. et al. Sin embargo, la incidência entre los contactos lepromino-negativos vacunados con Mycobacterium w, lue significativamente más haja que la observada entre los contactos lepromino-negativos que no fueron vacunados on los oiros dos estúdios (14.1% por Dharmendra, et al. y 29.0% por Chaudhary, et al.) En conclusion, aunque el estúdio se hizo con una población pequeña, los resultados preliminares indican que la vacunación con Mycobacterium w parece lener cl potencial de reducir la incidência de lepra entre los contactos familiares de los pacientes. Actualmente se realizan estúdios de campo en Kanpur Dehat que darán resultados más explícitos sobre la vacuna.The immunotherapeutic clinical trials with the candidate antileprosy vaccine based on a saprophytic, cultivable, rapid- growing rnycobacterium, Mycobacterium w, which commenced in 1986, have been completed and are under process of final reporting. Along with the immunotherapeutic trial in multibacillary (MB) leprosy patients, a study was also conducted in a small sample of the healthy household contacts (HHCs) of the index MB leprosy patients registered in the trial. The initial results of this study pertaining to the disease prevalence among the household contacts at initial screening, their lepromin status at the time of induction, and subsequent conversion to lepromin positivity, following administration of a varying number doses of Mycobacterium w vaccine among lep- romin-negative HHCs, have been reported earlier (7). However, in this communication we report 7-8 years of follow up of HCCs, including the final results of lepromin status and the incidence of new cases with disease among the HHCs.
MATERIALS AND METHODS
The subjects of this study were household contacts of the index patients with MB leprosy. Initially, a total of 362 contacts were screened, of whom 54 (14.9%) had active disease and were excluded. The remaining 308 apparently healthy household contacts were inducted in the study (7). Subsequently, 125 more HHCs were inducted into the study, thus bringing the total to 433 HHCs.
Lepromin tests. Lepromin tests were done using armadillo-derived lepromin (containing 30-40 million killed bacilli per ml), kindly made available by IMMLEP/ TDR of the World Health Organization (WHO) (Lot No. C-l, preparation date 6/14/89, NHDC, Carville, Louisiana, U.S.A.). The Mitusda response was recorded 3-4 weeks after the intradermal injection of lepromin-A. The lepromin response was graded as negative (3 mm or less), 1 + (4-6 mm), 2+ (7-9 mm) and 3+ (10 mm or above or response of any size with ulceration). Among those with a lep- romin-negative response retesting was done 3 months after each vaccine dose and subsequently every 6 months to monitor the stability of lepromin conversion.
Vaccine administration. The vaccine used was a suspension of killed Mycobacterium w in physiological saline in the concentration of 1010 bacilli per ml. The details of the vaccine preparation have been reported earlier (9). The first vaccine dose was 1 x 109 autoclaved bacilli in 0.1 ml physiological saline (0.85% NaCl). Subsequent doses, containing half the number of bacilli (5 x 108) were administered to the HHCs at intervals of approximately 3—4 months until conversion to lepromin posi- tivity was noticed. The vaccine was administered intradermally in the deltoid region, using a disposable 1 -ml syringe with a 30G needle.
RESULTS
Of the 433 apparently healthy contacts inducted, 374 contacts could be followed up clinically for periods ranging from 2-11 years, with an average follow-up period of 7.33 years. The remaining 59 HHCs could not be followed up, mainly because most of these contacts belonged to those index cases who themselves dropped out from the main immunotherapeutic study. Among the 374 HHCs followed up clinically, 231 were lepromin positive and 113 were lepromin negative on initial testing; the lepromin status of 30 HHCs could not be ascertained.
Initial lepromin status of 487 contacts (both apparently healthy and those with disease at outset). The initial lepromin status of 444 contacts out of the total 487 (including 433 apparently healthy and 54 with disease) is shown in Table 1. Of the 444 contacts, 302 (68%) were lepromin positive and 142 (32%) were lepromin negative on initial testing. The lepromin status of the remaining 43 contacts could not be assessed, either due to non-administration (unwilling subject) or contact did not return for a reading 3-4 weeks after lepromin administration.
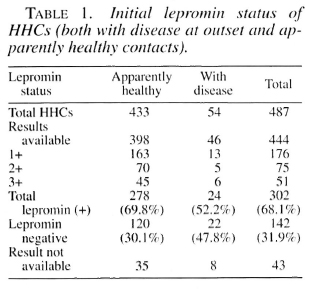
Lepromin status and disease type in 54 contacts with disease at outset. Of the 54 contacts with disease at outset, the initial lepromin status of 46 could be ascertained, of whom 22 (48%) cases were lepromin negative and 24 (52%) were lepromin positive. Of the 24 lepromin positives, 22 (92%) had paucibacillary (PB) leprosy and 2 (8%) had MB leprosy. Among the 22 lepromin negatives, the corresponding figures were 12 (54%) with PB and 10 (45%) with MB leprosy (Table 2).
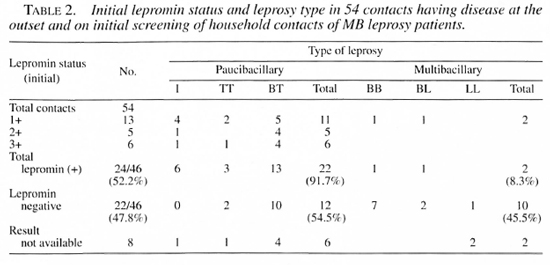
Course of lepromin status of lepromin negative HHCs administered Mycobacterium w vaccine. Of the 113 initially lep- romin-negative contacts, Mycobacterium w vaccine could be administered to 93 contacts. The remaining 20 lepromin-negative contacts did not receive this vaccine for various reasons and were followed up as such serving as the control group although of small sample size. The lepromin status follow up of 72 out of the 93 HHCs administered the Mycobacterium w vaccine (the remaining 21 contacts could not be retested for reasons similar to those mentioned above) is shown in Table 3. It may be noted that as many as 53 (74%) lepromin-nega- tive HHCs converted to positivity after a single dose of Mycobacterium w vaccine. The cumulative percentage conversion after the second and third doses was 87% and 93%, respectively. A total of 5 (7%) contacts remained lepromin negative for different durations of observation and after various numbers of doses (data not shown). One such case remained lepromin negative for 7.6 years even after receiving 6 doses of the vaccine; another still remained negative after 4 doses.
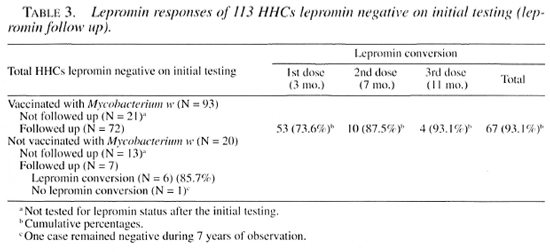
Lepromin status of HHCs not administered Mycobacterium w vaccine. Out of 20 lepromin-negative HHCs not administered the Mycobacterium w vaccine, lepromin response follow up was available for 7 cases. Of these, 6 converted to lepromin positivity spontaneously (observed in 3 cases after periods of 2.78, 3.52, and 6.65 years), and 1 case remained lepromin negative for an observation period of 7 years. Lepromin retesting could not be done in the remaining 13 cases.
New case detection among household contacts. The incidence of new cases with active disease in the 374 HHCs followed up clinically is shown in Table 4. A total of 8 new cases (3.5%) were detected from a total of 231 initially lepromin-positive HHCs.
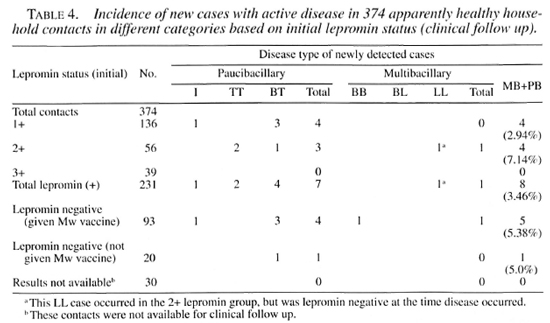
Of these, 4 were from 1+ grading (3 BT and 1 indeterminate) and 4 from 2+ grading (2 BT, 1 TT and 1 LL). No case was detected among 39 contacts with a 3+ grade lepromin response. Among 93 initially lepromin-negative HHCs administered Mycobacterium w, a total of 5 new cases (5.4%) were detected during follow up (3 BT, 1 indeterminate and 1 BB). Among 20 lepromin-negative contacts not administered the Mycobacterium w vaccine, 1 case (BT) was detected. Among 30 HHCs with an unknown initial lepromin status, no new case was detected.
DISCUSSION
The prognostic value of the lepromin test has been demonstrated in a number of studies over the years, and conventionally it has been considered a useful marker of protection against development of MB leprosy (3). The reliability of the lepromin status as such a marker has been reviewed in many studies, and in some of them correlation of lepromin status to the protection imparted has been viewed with reservations (4). In evaluating the immunoprophylactic ability of an immunomodulator in leprosy, the only reliable criteria for the effectiveness of a vaccine has been considered to be the actual reduction in number of newly detected cases among the vaccinated, apparently healthy contacts (2).
Among lepromin-positive contacts, the incidence of new cases in our study is 8/231 (3.5%) which is comparable to the observations of 17/524 (3.2%) in a similar group by Dharmendra, et al. (3). In another study of a similar nature by Chaudhary, et al. from Calcutta using three different vaccines, the incidence of new cases among lepromin- positive HHCs was 35/504 (6.9%) without vaccine (1).
Among lepromin-negative contacts, 14.1% (22/156) developed the disease in the study by Dharmendra, et al. while 29.0% (61/210) developed disease in the study by Chaudhary, et al. among contacts not receiving vaccine. However, the incidence among those receiving vaccine was brought down to 4.2% (2/48) in the latter study. In our study, the incidence was 5.4% (5/93) among lepromin-negative HHCs administered Mycobacterium w vaccine, and 5% (1/20) among the lepromin-negative contacts who did not receive the vaccine. The statistical difference of the last two groups is not significant (p = 0.630, chi squared test) but not much reliance can be assigned to this comparison because of the unbalanced sample sizes, i.e., 93 in the first group and only 20 cases in the second group. Since this smaller group was not planned initially, we tried to compare our study observations with a few other studies of a similar nature with respect to the disease incidence among household contacts. Obviously, such a comparison is prone to be affected by confounding factors due to dissimilarities in the designs of the different studies, and different conditions, but in the absence of any valid comparable sample this is being done for academic interest. The incidence of new cases with disease among lepromin-negative contacts administered Mycobacterium w vaccine in our study was 5.4%, as compared to 29% among those not administered any vaccine in the Calcutta study. A similar comparison between the results from our study (5.4% incidence) and those obtained by Dharmendra, et al. (14.1% incidence) is also of interest. In a recently reported, large-scale comparative field trial employing three different vaccines-BCG + killed M. leprae, ICRC and Mycobacterium w-administered in a single dose to a general population in an area of high endemicity, the Mycobacterium w vaccine has been shown to impart a protection of about 27%-30% in a sample of 38,000 people in a general population (6).
The type of disease acquired by the contacts in the two studies also provide some interesting observations. In the study by Dharmendra, et al., no case of lepromatous leprosy was detected among the initially lepromin-positive contacts. However, one case with this type of leprosy was observed in a lepromin-negative case who subsequently converted to positivity. In our study one contact developed lepromatous (LL) leprosy who was initially lepromin positive (2+). However, when he developed the discase alter a gap of 8 years, interestingly lie was lepromin negative, suggesting an unstable immune status in those with milder delayed-type hypersensitivity (DTH) responses to M. leprae. The other MB disease case, a contact who was lepromin negative initially and had received the vaccine, was diagnosed as BB leprosy. This contact remained lepromin negative alter two doses of the vaccine until the disease was detected. He converted to positivily after the third dose, after acquiring the disease. No case was detected among 39 contacts who had a lepromin response of 3+ at the initial testing, suggesting thereby that a strong DTH response is indicative of stable protective immunity.
Lepromin status in general seems to influence the type of leprosy developing in the contacts, as shown in Table 2. Among the contacts found to be having disease at the onset on initial screening, the lepromin response was negative in 47.8%. weakly positive (1+ or 2+) in 33.3% (18/54). while only 11.1% (6/54) had a lepromin response of 3+. 1 lowever. the occurrence of 2 IT and 10 BT cases with an initial lepromin-nega- tive status shows that there are times when the lepromin status does not coincide with the type of leprosy. Similarly, among the initial lepromin-positive contacts, one case each of BB and BL leprosy is present which, again, is an anomalous observation considering the characteristic lepromin response of these two types. These observations reinforce the opinion expressed in some studies (4) about the reliability of the lepromin response as a marker for the leprosy type and protection, with limited applicability and not a fool proof criterion which should be used as a broad guide only.
The rate of lepromin conversion among lepromin-negative contacts in the study by Chaudhary. et al. was around 73% after the first dose, which is similar to our observations (Table 3). The testing doses of lepromin-A may exert an immunomodulatory effect leading to spontaneous conversion to lepromin positivity (commonly referred to as microvaccination). In the group of contacts not administered the Mycobacterium w vaccine, such intervening immunomodulatory impact would make it difficult to compare and interpret the conversion to lepromin positivity attributable to the vaccine alone. However, it may be noted that such spontaneous conversions in our study have been observed altera considerable period (2.7-6 years) from the initial testing, during which the lepromin testing was clone for 4-5 times. On the other hand, the lepromin conversions following vaccine administration were noticed within 3-11 months, after 1, 2 or 3 closes in most of the cases (Table 3). This suggests that the quantum of impact of vaccination on lepromin conversion would outweigh that due to the microvaccination effect of lepromin-A, although the exact magnitude of this factor would be difficult to ascertain in the present study design. The sensitization potential of Mycobacterium w vaccine in a general population also has been evaluated in a large-scale field trial in the Chingelput district of South India (5), where the vaccine in a single close of 5 x 109 bacilli has been shown to produce significant sensitizing effects as measured by post-vaccination reaction to Rees' MLSA and lepromin preparation. No significant response however was noted in the close of 109.
The number of subjects lost to follow up in the long drawn studies of this nature is always a concern, and efforts are mandatory to reduce it to the lowest possible. In our study, 59 out of 433 contacts (13.6%) could not be followed up, primarily because of noncompliance of the index patients (whose contacts were being studied) in the main immunotherapeutic study (8). The drop-out rates of our study are comparable to those of another such study with a similar theme from South India, where the reported drop outs in the first and second survey have been 13.8% and 24.4%, respectively (6). Based on the initial examination of all contacts, the contacts lost to follow up did not have any different characteristics as compared to those who were followed up.
To conclude, the comparison of the results of the three different studies with similar objectives shows that the new disease incidence is similar among the lepromin- positive contacts, i.e., 3.2% by Dharmendra, et al., 6.9% by Chaudhary, et al. and 3.5% in the present study. Among lepromin-negative contacts not receiving any kind of immunomodulatory intervention, the incidences in the other two studies were 14.1 % (Dharmendra. et al.) and 29.0% (Chaudhary, et al.). However, the incidence is brought down considerably with immunomodulatory intervention among lep- romin-negative contacts, as shown by the incidence of 4.2% in the Calcutta study and 5.4% in the present study.
REFERENCES
1. Chaudhary, S., Hazra, S. K., Saha, B., Mazumdar, B., Biswas, P. C., Chattopadhya, D. and Saha, K. An eight year field trial on antileprosy vaccines among high-risk household contacts in the Calcutta metropolis. Int. J. Lepr. 62 (1994) 389-394.
2. Convit, J., Aranzazu, N., Ulrich, M., Zunig M., de Aragon, M. L., Alvarado, J. and Reyes, O. Investigations related to the development of a leprosy vaccine. Int. J. Lepr. 51 (1983) 531-539.
3. Dharmendra and Chatterjee, K. R. Prognostic value of lepromin test in contacts of leprosy cases. Lepr. India 27 (1955) 149-157.
4. Gupte, M. D., Anantiiaraman, D. S., De Briito, R. L. J., Vallishayee, R. S., Nagaraju, B., Kannan, S. and Sengupta, U. Sensitization potential and reactogenicity of BCG with and without various doses of killed Mycobacterium leprae. Int. J. Lepr. 60(1993)340-352.
5. Gupte, M. D., Vallishayee, R. S., Anantharaman, D. S., De Briito, R. L. J. and Nagaraju, B. Sensitization and reactogenicity of two doses of candidate anti-leprosy vaccine Mycobacterium . Indian J. Lepr. 68 (1996) 315-324.
6. Gupte, M. D., Vallishayee, R. S., Anantharaman, D. S., Nagaraju, B., Sreevatsa, S., Bala- subramanyam, S., De Briito, R. L. J., Elango, N., Uthayakumaran, N., Mahalingam, V. N., Lourdusamy, G., Ramalingam, A., Kannan, S. and Arokiasamy, J. Comparative leprosy vaccine trial in South India. Indian J. Lepr. 70 (1998) 369-388.
7. Kar, H. K., Sharma, A. K., Misra, R. S., Za- iieer, S. A., Mukherjee, A., Mukherjee, R., Beena, K. R., Kaur, H., Nair, S. and Talwar, G. P. Induction of lepromin positivity by a candidate anti-leprosy vaccine Mycobacterium w in lep- romin-negative household contacts of multibacil- lary leprosy patients. Indian J. Lepr. 64 (1992) 495-500.
8. Sharma, P., Kar, H. K., Misra, R. S., Mukherjee, A., Kaur, H., Mukherjee, R. and Rani, R. Induction of lepromin positivity following im- muno-chemotherapy with Mycobacterium w antileprosy vaccine and its impact on bacteriological clearance in MB leprosy. Int. J. Lepr. 67 (1999) 259-269.
9. Talwar, G. P., Zaheer, S. A., Mukherjee, R., Walia, R., Misra. R. S., Sharma, A. K., Kar, H. K., Mukherjee, A., Parida, S. K., Suresh, N. R., Nair, S. K. and Pandey, R. M. Immunotherapcu- tic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w, in multibacillary leprosy patients. Vaccine 8 (1990) 121-129.
1. D.V.D.; National Institute of Immunology, New Delhi 110 067, India.
2. Ph.D., National Institute of Immunology, New Delhi 110 067, India.
3. M.D., Department of Dermatology, Venereology and Leprology, Dr. Ram Manohar Lohia Hospital, New Delhi 110 001, India.
4. M.D., Department of Dermatology and Leprology, Safdarjung Hospital, New Delhi 110 029, India.
5. M.D., Institute of Pathology, Indian Council of Medical Research, Safdarjung Hospital Campus, New Delhi 110 029, India.
6. Ph.D., Dabur India Limited, 22 Site IV, Sahibabad, Ghaziabad, U.P., 201 010, India.
Reprint requests to Dr. Rajni Rani, Neuroimmunology Division, National Institute of Immunology, JNU Complex, New Delhi 110 067, India. FAX: 91-11-6162125; email: rajni@nii.res.in
Received for publication on 18 October 1999.
Accepted for publication in revised form on 6 April 2000.