- Volume 68 , Number 2
- Page: 129–35
Trials of daily, long-term minocycline and rifampin or clarithromycin and rifampin in the treatment of borderline lepromatous and lepromatous leprosy
ABSTRACT
Daily, long-term treatment with minocycline 100 mg and rifampin 600 mg was initiated in 24 previously untreated borderline lepromatous (BL) and lepromatous (LL) patients for a total of 646 patient-months, averaging 26.9 months per patient. The same regimen was started in 12 BL and LL patients having a bacteriologic relapse for a total of 379 patient-months, averaging 32.5 months per patient, and in 12 patients judged to be at high risk for relapse for a total of 354 patient-months, averaging 29.5 months per patient. Daily, long-term treatment with clarithromycin 500 mg and rifampin 600 mg was initiated in 8 previously untreated BL and LL patients for a total of 174 patient-months, averaging 21.8 months per patient. The results in these 56 patients were compared to those obtained in 34 previously untreated BLand LL patients who were treated concurrently receiving daily, long-term dapsone 100 mg and rifampin 600 mg. No evidence of dangerous drug reactions or bone marrow, kidney or liver toxicity was seen in any of these five patient groups. Drug intolerance in 10 of the 90 patients studied necessitated discontinuing the chosen regimen, 4 f rom rifampin, 3 f rom dapsone, 2 f rom minocycline and 1 of undetermined attribution. The use of either minocycline or clarithromycin in conjunction with rifampin appears to pose no great risk when used long term.RÉSUMÉ
Un traitement journalier au long cours associant 100 mg de minocycline et 600 mg de rifampine fut administre' chez 24 patients lépromateux (LL) et lépro- matcux borderline (BL), totalisant 646 patients x mois, soit une moyenne de 26,9 mois par patients. Le même traitement fut initié I ) chez 12 patients BLet LL ayant montré une rechute, prouvée à l'examen bactério- scopique, totalisant 379 patients x mois, soit une moyenne de 32.5 mois par patients et 2) chez 12 patients jugés être à fort risque de rechute, totalisant 354 patients x mois, soit une moyenne de 29.5 mois par patients. Un traitement, également caractérisé par des doses journalières associant 500 mg de clarithro- mycine et 600 mg de rifampine fut administré en premier traitement chez 8 patients BL et I.L, totalisant 174 patients x mois, soit 21.8 mois en moyenne par patient. Les résultats de ces 56 patients furent comparés avec ceux obtenus à partir de 34 patients traités en première intention par des doses journalières à long terme associant 100 mg de dapsone et 600 mg de rafampine. Aucun signe de réactions médicamenteuses sérieuses on de toxicité médullaire, rénale ou hépatique ne fut détecté, quelque soit le groupe étudié. Une intolérance médicamenteuse, observée chez 10 de ces 90 patients étudiés, a nécessité l'interruption du traitement sélectionné. 4 provenant d'une intolérance à la rifampine, 3 à la dapsone, 2 à la minocycline et 1 intolerance d'origine indéterminée. L'utilisation de la minocycline ou de la clarithromycine en complément de la rifampine ne semble pas poser de risque séerieux, lorsquie utilisé à long terme.RESUMEN
Diariamente se administraron 100 mg de minociclina y 600 mg de rifampina a 24 pacientes con lepra lepromatosa subpolar (BL) o lepra lepromatosa (LL) sin tratamiento prévio, por un total de 646 paciente-meses (26.9 meses por paciente). El mismo tratamiento fue administrado a 12 pacientes BL y LL con evidencias bacteriológicas de racaída, por un total de 379 paciente-meses (32.5 meses por pacient), y a 12 pacientes en alto riesgo de recaída, por un total de 354 paciente-meses (29.5 meses por paciente). Por otro lado, diariamente se adminstraron 500 mg de claritro- micina y 600 mg de rifampina a 8 pacientes BL y LL sin tratamiento prévio, por un total de 174 paciente- meses y un promedio de 21.8 meses por paciente. Los resultados en estos 56 pacientes fueron comparados con los obtenidos en 34 pacientes BLy LLque fueron tratados con 100 mg de dapsona y 600 mg de rifampina. por períodos similares de tiempo. En ninguno de estos 5 grupos de pacientes se observaron evidencias de reacciones peligrosas, ni de toxicidad en medula ósea, rinón o hfgado. Sin embargo, 10 de los pacientes estudiados mostraron intolerância a alguna de las drogas utilizadas. Los casos de intolerância fueron a la rifampina (cuatro), a la dapsona (tres), a la minociclina (dos), y uno de atribución indeterminada. El uso de minociclina o de claritromicina junto con rifampina parece no representar mayor riesgo cuando se usa en tratamientos de larga duración.In 1970 rifampin was the first antibiotic demonstrated to be strongly microbiocidal for Mycobacterium leprae in humans (18). In a short period of time in the 1990s, three additional antibiotics were recognized as also having potent bactericidal activity against M. leprae in humans, specifically minocycline (4,9), clarithromycin (9), and several of the fluoroquinolone group ( 5), with ofloxacin receiving the most attention (10-12, 14) These sudden advances provided opportunities to explore new therapeutic regimens in the treatment of leprosy. The motivation for such exploration comes from several sources. For example, a shorter period of time for treatment would reduce noncompliance and perhaps costs as well. Also, the high relapse rate observed by Jamet, et al. (8) among heavily bacillary patients treated with the conventional combination of rifampin, dapsone and clofazimine suggested a need for alternative regimens. Finally, combinations of microbiocidal agents might be better than combinations of microbiocidal drugs which also include a bacteriostatic agent, reasoning a priori as well as by analogy with the treatment of human tuberculosis, for which the use of multiple bactericidal agents has become standard (6).
Reported in the present paper is the long- term experience with two combinations of bactericidal agents, minocycline and rifampin, or clarithromycin and rifampin, in the management of lepromatous (LL) and borderline lepromatous (BL) leprosy. To address questions of their safety, tolerance, and effect upon the reactional state frequency, patients receiving these combination bactericidal regimens were compared with those treated concurrently with dapsone and rifampin.
MATERIALS AND METHODS
Patients in the Hansen's Disease Clinic of the Los Angeles County/University of Southern California Medical Center, Los Angeles, California, U.S.A., were classified according the criteria and nomenclature of Ridley and his colleagues (19). Criteria for the diagnosis of reactional states were as previously reported for erythema nodosum ieprosum (ENL) (19), delayed-type hypersensitivity (DTH) reactions (17), and the Lucio reaction (16).
Whatever the regimen used, patients were encouraged to stay on combination therapy for a period of 3-3½ years, followed by single drug therapy indefinitely. However, each patient was offered the opportunity to cease drug treatment after 2 years. Of the patients reported in this study, none elected to stop treatment altogether after 2 years, but one did stop combined therapy after 2 years but chose to continue with a single agent.
The regimens employed consisted of 1) minocycline 100 mg daily and rifampin 600 mg daily, 2) clarithromycin 500 mg daily and rifampin 600 mg daily, and 3) dapsone 100 mg daily and rifampin 600 mg daily. In the event of a DTH reaction managed with corticosteroids, rifampin was sometimes reduced to 600 mg monthly or, if very severe, discontinued altogether. At its commencement dapsone therapy was initiated at 25 mg daily, increased by 25 mg increments at 7 day intervals, and monitored at weekly intervals for the first 4 weeks. Similarly, initial rifampin use was monitored at intervals of 1-2 weeks for the first 4 weeks. After 4 weeks, patients were routinely seen at 3-month intervals, more often if required by reactional states.
A blood count and blood chemistries were obtained before starting treatment and at each subsequent clinic visit. Normal values for leukocytes for men were 4.9-9.9 x 103/mm3; for women, 4.2-12.3. The normal values for tests reflecting liver function were as follows: bilirubin, total 0.0-1.0 and direct 0.0-0.3 mg/dL; alkaline phosphates, 45-140 units/dL; lactic acid dehydrogenase (LDH), 90-220 units/dL; aspartate aminotransferase (AST), 10-40 units/dL; and alanine aminotransferase (ALT), 20-65 units/dL. Renal function was monitored by including creatinine and urea levels in the blood chemistries.
A leukopenia, if present for three consecutive counts, was considered to be "persistent," but if occurring less often, was considered to be "sporadic." An anemia of 10 g/dL of hemoglobin or less was considered to be a contraindication to starting dapsone and a reason to discontinue it, if occurring early in therapy and not associated with ENL.
Concerning possible adverse hepatic reactions, liver function tests were considered to be "normal" if no more than one abnormality occurred yearly, were judged to be "persistently abnormal" if abnormal on three consecutive visits, but were judged to be "sporadically abnormal" if more than one abnormality per year was seen but not on three consecutive visits.
No patients were enrolled in this study after 30 June 1999. All data considered in this study were recorded before 1 January 2000.
RESULTS
A total of 56 patients, in four different groups, received a combination microbiocidal regimen. Daily minocycline and rifampin was started in 24 previously untreated BL or LL patients for a total of 646 patient-months, an average of 26.9 months per patient. Daily clarithromycin and rifampin was started in eight previously untreated BL or LL patients for a total of 174 patient-months, an average of 21 .8 months per patient. In addition, daily minocycline and rifampin was started in 12 BL or LL patients who had a bacteriologic relapse during dapsone therapy or after prolonged noncompliance, and in 12 BL or LL patients who were judged to be at risk of relapse, because of frequent failure to keep clinic appointments, for 379 and 354 patient- months, an average of 32.5 and 29.5 months per patient, respectively. Concurrently with these four groups, a fifth group, consisting of 34 previously untreated BLor LL patients, received daily dapsone and rifampin for a total of 870 patient-months, an average of 25.6 months per patient.
Demographic and leprologic data for the three previously untreated groups are summarized in Figure I. With the exceptions noted below, the three groups were comparable. The low percentage of Mexican-born patients in the group receiving clarithromycin and rifampin reflects the diminished number of such individuals in our clinic in 1997 and 1998, the years in which most in the group were recruited. The higher percentage of LL patients receiving minocycline and rifampin, as well as the higher percentage of patients presenting in reaction who received either minocycline or clarithromycin in combination with rifampin, reflects an enrollment bias based upon the supposition, later shown to be incorrect, that the anti-inflammatory properties of these agents might reduce the incidence or severity of reactions. The reactions present at the time of presentation were ENL, DTH and Lucio. The relatively higher incidence of ENL developing in previously untreated patients after receiving minocycline and rifampin reflects the relative preponderance of LL patients in this group. The patients' ages in these three groups, as judged by oldest, youngest, and median ages, were also similar (data not shown).
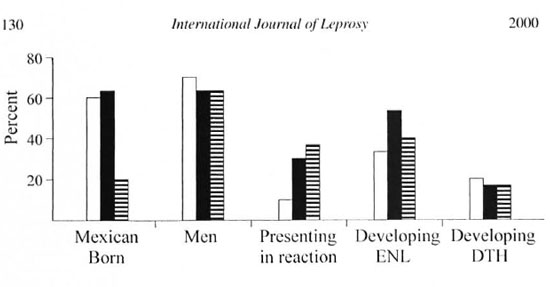
Fig. 1. A graphic representation, expressed as percentages, of patients born in Mexieo, patients who were men, patients who presented im reaction (including ENL, DTH, and Lucio), patients who developed ENL after starting treatment, and patients who developed DTH after starting treatment, as occurring in the three groups of patients who had no prior treatment.  = Dapsone + rifampin:
= Dapsone + rifampin:  = minocycline + rifampin:
= minocycline + rifampin:  = clarithrontycin + rifampin.
= clarithrontycin + rifampin.
Concerning attrition, among all 90 patients in the five groups studied, 24 failed to complete 24 months of their combined mi- crobiocidal regimen. Of these 24 failures, 15 were not related to any adverse drug experiences: 9 moved away, 3 died, 2 developed difficult to manage DTH reactions, and 1 became pregnant. In 9 the failure was attributed to (not rigorously proven to be) an adverse drug reaction, 4 from rifampin (2 persistent headaches, 1 eruption, and 1 itching), 3 from dapsone (1 anemia with a hemoglobin of <10 g/dL, 1 possible neuropathy, and 1 eruption), 1 from minocycline (hyperpigmentation) and 1 of undecided attribution (tremulousness while receiving minocycline and rifampin). From 24 months onward failure to complete a recommended 36 months of treatment occurred in an additional 4 patients; 1 adverse drug reaction (hyperpigmentation from minocycline), 2 patients moved and 1 became pregnant. Among the three previously untreated groups, no adverse reaction requiring discontinuance of the regimen was seen in the small clarithromycin and rifampin group. The incidence of adverse drug reactions in the other two previously untreated groups was similar, 17% in those receiving minocycline and rifampin and 12% in those receiving dapsone and rifampin, and did not differ significantly from that of the relapsed and risk of relapse groups, 8% in each. Minocycline-induced hyperpigmentation, to a degree that some patients would find objectionable, was seen in an additional eight patients, individuals who happened to be not bothered by this adverse response. Thus, a total of 10 of 47 patients receiving minocycline had appreciable hyperpigmentation. None of the three deaths, one in each of the three previously untreated groups, appeared to be medication related (an adenocarcinoma of the lung first identified by the routine pre- treatment clinic chest X-ray, an adenocarcinoma of the colon, and a dissection of an aortic aneurysm).
Concerning peripheral blood values, in the initial 6 months of therapy in previously untreated patients receiving minocycline and rifampin, the mean hemoglobin values increased from a baseline of 13.5 to 14.1 g/dL, p <0.002, t test of paired samples for means (N = 18). In contrast in those previously untreated patients receiving dapsone and rifampin, the mean hemoglobin values fell from a baseline of 14.6 to 13.1 g/dL, p <0.0002 (N = 29). No statistically significant increase was found in the group receiving clarithromycin and rifampin, probably because of the small sample size (data not given).
Concerning leukocytes, some leukopenia was found in 24 of 84 patients treated long enough to evaluate, and leukopenia was present in all five treatment groups. In 13 of these 24 patients the leukopenia was sporadic. Persistent leukopenia, found in 11 patients, was present in all five treatment groups without any statistically significant differences among them. Eight of these 11 had a baseline leukopenia, indicating that the combination microbiocidal therapy was not responsible. Also, thalidomide, an agent recognized to be associated with leukopenia (7), was used in 5 of these 11 patients. No abnormal differential counts were found. In no patient was the antibiotic regimen changed because of leukopenia. With two exceptions the leukocytosis observed in 16 patients was associated with ENL or the use of corticosteroids to treat DTH reactions, but no explanation was ever found in the two exceptions.
Figure 2 summarizes the abnormal liver function tests observed in each of the five patient groups. The group judged to be at risk of relapse had a particularly high incidence of baseline abnormalities, perhaps a concomitant of the life style associated with noncompliance. Baseline abnormalities were present in 21 of the 90 patients, but in 10 no subsequent sporadic or persistent abnormalities could be found. There was no statistically significant difference in the incidence of sporadic or persistent abnormalities observed in any treatment group. The abnormalities found were usually mild elevations and did not result in altering any antibiotic regimen used.
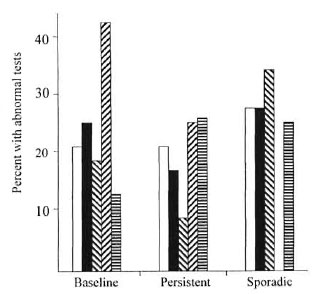
Fig. 2. Percent of patients having some serum abnormality indicating abnormal liver function in each of the live treatment groups, as occurring before initiating dual microbiocidal therapy or after starting therapy and occurring either sporadically or persistently.  = Dapsone + rifampin, no prior treatment;
= Dapsone + rifampin, no prior treatment;  = minocycline + rifampin, no prior treatment;
= minocycline + rifampin, no prior treatment;  = minocycline + rifampin, bacteriologic relapse;
= minocycline + rifampin, bacteriologic relapse;  = minocycline + rifampin, risk of relapse;
= minocycline + rifampin, risk of relapse;  = clarithromycin + rifampin, no prior treatment.
= clarithromycin + rifampin, no prior treatment.
Unless diabetic nephropathy was present, all blood creatinine levels were normal, indicating no nephrotoxicity from any of the drug combinations used.
No bacteriologic relapses have been found in any of the previously untreated patients enrolled in this study.
DISCUSSION
The long term use of two combination microbiocidal regimens-daily minocycline and rifampin, and daily clarithromycin and rifampin-in the treatment of multibacillary (MB) leprosy has been found to be safe and well tolerated, as judged by comparison with concurrently treated patients receiving daily dapsone and rifampin.
Regarding safety, 48 patients receiving minocycline and rifampin, as well as eight receiving clarithromycin and rifampin, had no serious evidence of bone marrow suppression, hepatic toxicity, or renal toxicity, nor any other serious drug reaction. Again regarding safety, i.e., dangerous drug reactions, leukopenia, and abnormal liver function tests, these patients differed little from 34 concurrently treated individuals receiving dapsone and rifampin. This conclusion must be tempered by the relatively small number of patients treated.
Drug intolerance sufficient to terminate a regimen, as contrasted to drug danger, occurred in response to dapsone, rifampin, and minocycline. It should be emphasized that the attribution of a drug effect was a clinical impression. The inferences of itching and an eruption from rifampin as well as neuropathy from dapsone are challengeable, but in the case of itching, it did recur when rifampin was restarted. Because our clinic has experienced two patients with persistent vomiting in association with clarithromycin use for paucibacillary (PB) leprosy, the absence of intolerance to clarithromycin in this study is most probably due to the small number of patients.
Minocycline-induced hyperpigmentation, first described at sites of cutaneous inflammation (2), has been previously reported in this pattern in a BL patient (3). Also, a diffuse hyperpigmentation has been especially carefully documented in patients treated with acne vulgaris or acne rosacea (1); in these patients the diffuse hyperpigmentation was not seen until 3 years of treatment, but was present in 50% of those treated for 3 years or longer. The pigments involved, as with clofazimine, appear to be multiple, including aggregates of minocycline (13 ),iron chelated with minocycline (13), and melanin, perhaps induced by activation of tyrosinase, secondary to iron accumulation (3). The pigments are found primarily in macrophages, hence their occurrence in sites of skin inflammation as well as in a generalized distribution in lepromatous leprosy, the clinically normal skin being diffusely infiltrated with macrophages (l5).
Concerning the patterns of minocycline- induced hyperpigmentation seen in the patients reported here, the patchy blue-black color found at lesion sites was the most common, being particularly apt to appear on the legs and feet. When the hyperpigmentation was diffuse or generalized, it appeared to be melanin or melanin-like, was most pronounced in sun exposed areas, and was seen within 6 months of initiation of treatment. Both patterns were sometimes seen in the same area, usually on the face. The "physiologic" appearance of minocy- cline-induced diffuse hyperpigmentation may be why it can be better tolerated than that from clofazimine. It is the uniform opinion of the dermatologists attending in this clinic that the hyperpigmentation from minocycline is far more common in leprosy than in acne vulgaris patients, perhaps a consequence of the large number of macrophages in leprosy, whether in or between the lesions, or perhaps a matter of dosage, acne vulgaris patients often receiving 50 mg daily once a remission is produced. As observed by others (1), the diffuse hyperpigmentation is very gradual in onset, hence difficult to perceive by patient and clinician alike. For this reason, and because mild minocycline-induced hyperpigmentation of the legs may be difficult to distinguish from hemosiderin, the exact incidence of minocycline-induced hyperpigmentation is probably under-reported in these patients.
The use of combination microbiocidal regimens did not alter the incidence of reactional states, nor appear to ameliorate the severity of ENL or DTH reactions occurring at the time of presentation. In two patients presenting with the Lucio reaction, new lesions did not occur following the immediate daily use of minocycline in one and clarithromycin in the other, rifampin not being started until 7 days later. This is similar to our findings with rifampin use in previously untreated Lucio reactions, and is also consistent with our speculation that the Lucio reaction has a requirement for viable organisms (unpublished observations).
This study provides no information on the effectiveness of long-term, combination microbiocidal regimens in MB leprosy as compared to the more common combination of microbiocidal and bacteriostatic agents. With all regimens using rifampin, both large numbers of patients and a long period of observation following completion of treatment would be required in order to identify differences. Because most of our untreated MB patients are heavily bacillary (BI >5.0) and because the risk of relapse in the Jamet report (8) was particularly great in patients with high Bis, trials of alternative regimens were considered to be important, even if only to establish safety and tolerance.
As practicing dermatologists our clinic staff has had extensive experience with long-term (2 years or more) use of minocycline or erythromycin, an agent very similar to clarithromycin, in the management of acne vulgaris. Hence, these two agents were considered to be good candidates for long- term trials in conjunction with rifampin. Because sizable long-term experience with fluoroquinolones is not available, these agents were not considered to be as well suited for daily, long-term trials.
The expense of minocycline and clarithromycin make them unlikely candidates for extensive use in heavily endemic areas with limited health care budgets. Based upon our experience, minocycline and clarithromycin might be of practical value in selected patients, such as those with anemia and cardiac problems which could be worsened with dapsone, or those with serious morbidity from drug intolerance.
Acknowledgment. Jeffrey Ashley, M.D., and Seth Vaccaro, M.D., provided most of the clinical care rendered to the subjects of this report. Mrs. Helen Mora, the clinic nurse, has created a hospitable clinic environment which enabled these studies to flourish. Any faults in this report are those of its author.
REFERENCES
1. Dwyer, C. M., Cuddihy, A. M., Kerr, R. E. I., Chapman, R. S. and Albam, B. F. Skin pigmentation due to minocycline treatment of facial dermatoses. Br. J. Dermatol. 129(1993) 158-162.
2. Fenske, N. A., Millns, J. L. and Greer, K. E. Minocycline-induced hyperpigmentation at sites of cutaneous inflammation. JAMA 244 (1980) 1103-1106.
3. Fleming, C. J.. Hunt, M. J., Salisburg, E. L. C., McCarthy, S. W. and Barnetson, R. St. C. Minocycline-induced hyperpigmentation in leprosy. Br. J. Dermatol. 134 (1996) 784-787.
4. Gelber, R. H., Fukuda, K., Byrd, S., Mukry, L. P., Siu, P., Tsang, M. and Rea, T. H. A clinical trial of minocycline in lepromatous leprosy. Br. Med. J. 304 (1992) 91-92.
5. Grosset, J. H., Ji, B. H., Guelpa-Lauras, C. C., Perani, E. G. and N'Deli, L. N. Clinical trial of pelloxaein and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58 (1990) 281-295.
6. Haas, D. W. Mycobacterium tuberculosis. In: Mandell, Douglass, anil Bennet's Principles and Practice of Infections Disease. 5th edn. Edinburgh: Churchill Livingston, pp. 2576-2607.
7. Hastings, R. C., Trautman, J. R.. Enna, C. D. and Jacobson, R. R. Thalidomide in the treatment of erythema nodosum leprosum with a note on selected laboratory abnormalities in erythema nodosum leprosum. Clin. Pharmacol. Ther. 11 (1970)481-487.
8. Jamet, P., Ji, B., and the Marchoux Chemotherapy Study Group. Relapse after long-term follow up of multibacillary patients treated by WHO multidrug regimen. Int. J. Lepr. 63 (1995) 195-201.
9. Ji. B.. Jamet, P., Perani, E. G., Bobin, I'. and Grosset, J. H. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J. Infect. Dis. 168(1993) 188-190.
10. Ji. B., Jamet. P., Perani, E. G., Sow, S.. Lien- hardt, C.. Petinon, C. and Grosset, J. H. Bactericidal activity of single dose of clarithromycin plus minocycline, with or without ofloxacin, against Mycobacterium leprae in patients. Antimi- crob. Agents Chemother. 40 (1996) 2137-2141.
11. Ji, B., Perani. E. G., Petinon, C. N'Deli, L. and Grosset, J. H. Clinical.trial of ofloxacin alone and in combination with dapsone plus clofazimine for treatment of lepromatous leprosy. Antimicrob. Agents Chemother. 38 (1994) 662-667.
12. Mane, I., Cartel, J. L. and Grosset, J. H. Field trial of efficacy of supervised monthly doses of 600 mg rifampin, 400 mg ofloxacin and 100 mg minocycline for the treatment of leprosy; first results. Int. J. Lepr. 65 (1997) 224-229.
13. Okada, N., Sato, S., Sason, T.. Aoyama, M., Nishida, K. and Yoshikawa, K. Characterization of pigmented granules in minocycline-induced cutaneous pigmentation: observations using fluorescence microscopy and high performance liquid chromatography. Br. J. Dermatol. 129 (1993) 403-407.
14. Rao. P. S., Ramachandran, A., Sf.kar, B., Ravi, S. and Subramanian, M. Ofloxacin-containing combined drug regimens in the treatment of lepromatous leprosy. Lepr. Rev. 65 (1994) 181-189.
15. Rea, T. H., Gottleib, B. and Levan, N. E. Apparently normal skin in lepromatous leprosy. Arch. Dermatol. 111(1975) 1571-1574.
16. Rea, T. H. and Levan, N. E. Lucio's phenomenon and diffuse non-nodular lepromatous leprosy. Arch. Dermatol. 114(1978) 1023-1028.
17. Rea, T. H. and Sieling, P. A. Delayed-type hypersensitivity reactions followed by erythema nodosum leprosum. Int. J. Lepr. 66 (1998) 316-327.
18. Rees, R. J. W., Pearson, J. M. H. and Waters, M. F. R. Experimental and clinical studies on rifampicin in treatment of leprosy. Br. Med. J. 688 (1970)89-92.
19. Ridley, D. S. Histological classification and the immunological spectrum of leprosy. Bull. WHO 51(1974)451-465.
M.D., Division of Dermatology, The Keck School of Medicine, University of Southern California and The Los Angeles County/University of Southern California Medical Center, Los Angeles, CA, U.S.A.
Reprint requests to T. H. Rea, M.D., Division of Dermatology, LAC/USC Medical Center, Room 8441, 1200 N. State Street, Los Angeles, CA 90033, U.S.A. e-mail: rea@hsc.uscedu
Received for publication on 5 April 2000.
Accepted for publication on 3 May 2000.